Electrochemical formation of ion-conducting oxo-phosphate-molybdate polymer on aluminium
Main Article Content
Abstract
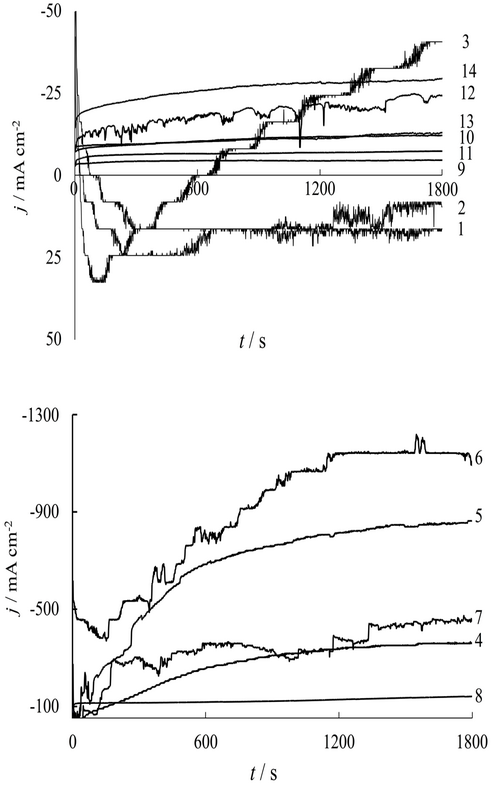
The state of theelectrochemical behavior of the Al electrode in aqueous solutions containing Na2MoO4 + H3PO4 + chitosan was investigated by methods of currentless chronopotentiometry, chronoamperometry, optical and scanning electron microscopy.The modification of the surface polymolybdate phosphate layers was carried out in a potentiostatic mode in the potentials rangefrom –1 to –3 V and polarization time (15…90 min). The elemental compositionof the surface layer of the metal before and after cathodic polarization are investigated.The electrochemical formation of matrix polymeric structures from ion-conducting (H+, Na+) heteronuclear polymolybdate and polyphosphate-molybdate complexes of double salts Na6AlnMo7–nO24and Na2yAl2(MoO4)y(PO4)3–ywas established.The rate of hydrogen and sodium intercalationinto the structure of the polyoxophosphate-molybdate layer increases sharply with an increase of the potential range from –1.3 V to –3.0 V. The addition of chitosan into the solution enhances the film-forming effect of surface ion-conducting oxo-phosphate – molybdate polymeric layer .
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
B. P. Tarasov, M. V. Lototskii, V. A. Yartys, Russ. J. Gen. Chem. 77 (2007) 694 (https://dx.doi.org/10.1134/s1070363207040329)
V. N. Ageev, I. N. Beckman, O. P. Burmistrova, Interaction of Hydrogen with Metals, Nauka, Moscow, 1987, p. 296 (https://b-ok.cc/ireader/3065144)
W. X. Chen, Int. J. Hydrogen Energy 26 (2001) 603 (https://doi.org/10.1016/S0360-3199(00)00119-1)
K. Young, J. Nei, Materials 6 (2013) 4574 (https://doi.org/10.3390/ma6104574)
S. S. Popova, L. A. Alekseeva, B. N. Kabanov, Russ. J. Electrochem. 22 (1986) 1427
S. S. Popova, N. A. Sobgaida, Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 45 (2002) 84
N. G. Krapivny, Russ. J. Electrochem. 17 (1981) 678
W. X. Chen, Intern. J. Hydrogen Energy 26 (2006) 603 (https://doi.org/10.1016/S0360-3199(00)00119-1)
A. V. Zvyaginceva, ISJAEE 21 (2015) 145 (https://doi.org/10.15518/isjaee.2015.21.018)
E. O. Chudotvorova, P. I. Bestuzhev, V. V. Kozlyakov, in Proceedings of The III International Scientific and Practical Conference, Minsk, 2016, Belarus, Collection of Abstracts, 2016, p. 43
A. V. Reznichenko, V. V. Rybalchenko, F. Z. Badaev, S. G. Ponomarev, A. A. Vasin, J Chem. Eng. Process. Technol. 8 (2017) 347(https://doi.org/10.4172/2157-7048.1000347)
Z. Ragaiy, L. Brenda, G. Diaz, C. S. Fewox, C. Ashley, J. Stowe, R. G. Gray, G. H. Andrew, Chem. Comm. 25 (2009) 3717 (https://doi.org/10.1039/B901878F)
C. C. Wang, Y. C. Chou, C. Y. Yen, Procedia Eng. 36 (2012) 105 (https://doi.org/10.1016/j.proeng.2012.03.017)
H. Z. Wang, D. Y. C. Zeung, M. K. H., M. Ni, Renew. Sustain. Energy Rev. 13 (2009) 845 (https://doi.org/10.1016/j.rser.2008.02.009)
H. Zou, , S. Chen, , Z. Zhao, W. Lin, J. Alloys Compd. 578 (2013) 380 (https://doi.org/10.1016/j.jallcom.2013.06.016)
R. Ripan, I. Chetyanu, Inorganic chemistry. Vol. 2. Chemistry of Metals, M publishing house Mir, Moscow, 1972, p.872
I. O. Grigorieva, A. F.Dresvyannikov, A. S. Zifirov, Bullet. Kazan Tech. Univ. 16 (2013) 271
A. Mueller, S. Roy, Usp. Khim. 71 (2002) 1101 (https://doi.org/10.1070/rc2002v071n12abeh000751)
N. A. Perekhrest, K. N. Pimenova, V. D. Litovchenko, J. Appl. Chem. 65 (1992) 1163
O. A. Stadnik, N. D. Ivanova, E. I. Boldyrev, L. I. Zheleznova, Ukr. Chem. J. 74 (2009) 55
E. E. Tekutskaya, I. Ya. Turyan, V. I. Kravtsov, V. V. Kondrat'ev, Russ. J. Electrochem. 27 (1991) 407
E. E. Tekutskaya, V. I. Kravtsov, Ind. Lab. Diagn. Mater. 64 (1998) 8
M. A Krayukhina. , N.A. Samoilova, I. A. Yamskov, Usp. Khim. 77 (2008) 854 (https://doi.org/10.1070/RC2008v077n09ABEH003750)
Ya. A. Kamenchuk, E. A. Zelichenko, V. V. Guzeev, Perspektivnye materialy 6 (2009) 66
V. Scheveleva, L. A. Zemskova, A. V. Voight, V. G. Kuryavy, Khimicheskie Volokna 2 (2008) 44
T. S. Khakamov, D. V. Feoktistov, L. A. Badykova, P. G. Kornilaev, R. R. Shavaleev, R. K. Mudarisova, Russ. J. Appl. Chem. 86 (2013) 1417 (https://doi.org/10.1134/S1070427213090175)
S. S. Popova, O. G. Kovalenko, V. V. Kurchavova, K. A. Belousov, Perspektivnye materialy 11 (2013) 35
S. S. Popova, H. A. Hussein, I. I. Frolova, V. F, Abdullin, Electrochem. Energetic 20 (2020) 99 (https://doi.org/10.18500/1608-4039-2020-20-2-99-111)
S. S. Popova, Methods of investigation of kinetics of electrochemical processes, Saratov State Technical University, Saratov, 2008, p.106
O. N. Lyubiev, Numerical methods in electrochemistry, NPI Publishing House, Novocherkassk, 1982, p.68
V. L. Mironov, Fundamentals of scanning probe microscopy: Textbook for senior students of higher educational institutions, Institute for Physics of Microstructures of the Russian Academy of Sciences, Nizhny Novgorod, 2004, p. 110
V. M. Zolotarev, N. V. Nikonorov, A. I. Ignatiev, Modern methods for the study of optical materials Part 2, ITMO University, St. Petersburg, 2013, p.166
J. I. Goldstein, H. Yahowitz, D. E. Newbury, E. Lilshin, J. W. Colby, J. R. Coleman, Practical Scanning Electron Microscopy: Electron and Ion Microprobe Analysis, Plenum Press, New-York, 1975, p.598 (https://doi.org/ 10.1007/978-1-4613-4422-3)
L. V. Baranova, E. L. Demina, Metallographic etching of metals and alloys: Handbook, Metallurgy, Moscow, 1968, p. 256
S. P. Chizhik, L. K. Grigorieva, R. N. Kuklin, Doklady Akad. Nauk SSSR 321 (1991) 1221.