Sodium ion chemosensor of 3-oxo-3H-benzo[f]chromene-2-carboxylic acid: An experimental and computational study Scientific paper
Main Article Content
Abstract
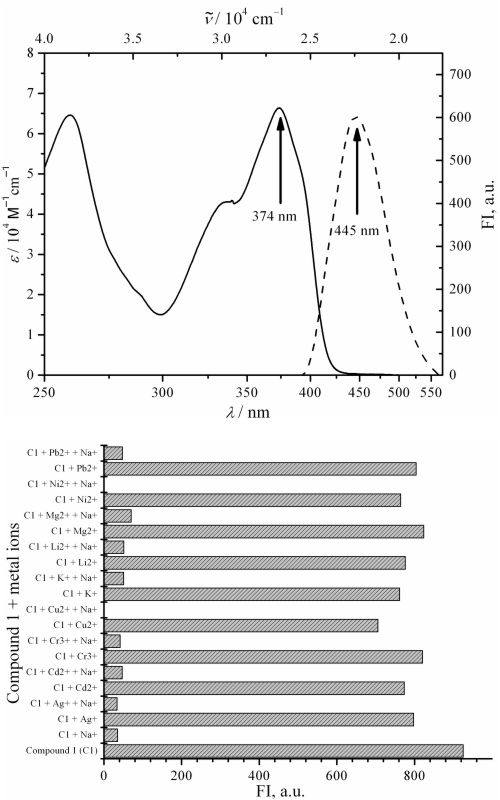
A fluorescence compound with the typical skeleton of benzocoumarin was synthesized and its interaction with various metal ions was evaluated. The synthesis was performed via Knoevenagel condensation whereas identification of the product was accomplished by various spectroscopic techniques. The chemosensor test against representative metal ions was monitored by fluorecence spectrophotometry. A density functional theory calculation (DFT, functional/basis set; M06/6-31G (d, p)) was also performed to clarify the experimental results and to confirm the mechanism of interaction. 3-Oxo-3H-benzo[f]chromene-2-carboxylic acid 1 was obtained as a yellow solid in 60 % chemical yield. Melting point; 235.6–236.7 °C and λmax UV/Vis, λem and Stokes shift (MeOH, nm) of 374, 445 and 71 nm, respectively. The structure of the compound was identified based on spectroscopic data and literature comparison. Compound 1 exhibited a chelation quenched fluorescence (CHQF) phenmenon selectively toward the Na+, with a binding stoichiometry (1:2) and LoD and LoQ of 0.14 and 0.48 mg/L, respectively. Based on DFT calculations, compound 1 chelated Na+ through mechanism of oxidative (1:1 equivalent) and reductive (2:1 equivalent) photoinduced electron transfer (PET), correspondingly.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
C. Zhou, N. Xiao, Y. Li, Can. J. Chem. 92 (2014) 1092 (https://dx.doi.org/10.1139/cjc-2014-0011)
J. S. Kim, D. T. Quang, Chem. Rev. 107 (2007) 3780 (https://dx.doi.org/10.1021/cr068046j)
H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim, J. Yoon, Chem. Soc. Rev. 37 (2008) 1465 (http://dx.doi.org/10.1039/B802497A)
X. Chen, T. Pradhan, F. Wang, J. S. Kim, J. Yoon, Chem. Rev. 112 (2012) 1910 (https://doi.org/10.1021/cr200201z)
J. F. Clark, D. L. Clark, G. D. Whitener, N. C. Schroeder, S. H. Strauss, Environ. Sci. Technol. 30 (1996) 3124 (https://dx.doi.org/10.1021/es960394n)
M. P. Anderson, R. J. Gregory, S. Thompson, D. W. Souza, S. Paul, R. C. Mulligan, A. E. Smith, M. J. Welsh, Science 80 253 (1991) 202 (https://dx.doi.org/10.1126/science.1712984)
Y. Yamini, N. Alizadeh, M. Shamsipur, Anal. Chim. Acta 355 (1997) 69 (https://dx.doi.org/10.1016/S0003-2670(97)81613-3)
C.F. Harrington, S.A. Merson, T. M. D. DSilva, Anal. Chim. Acta 505 (2004) 247 (https://dx.doi.org/10.1016/j.aca.2003.10.046)
S. L. C. Ferreira, A. S. Queiroz, M. S. Fernandes, H. C. dos Santos, Spectrochim. Acta, B 57 (2002) 1939–1950 (https://dx.doi.org/10.1016/S0584-8547(02)00160-X)
J. C. Yu, J. M. Lo, C. M. Wai, Anal. Chim. Acta 154 (1983) 307 (https://dx.doi.org/10.1016/0003-2670(83)80032-4)
A. Ali, H. Shen, X. Yin, Anal. Chim. Acta 369 (1998) 215 (https://doi.org/10.1016/S0003-2670(98)00252-9)
A. Bobrowski, K. Nowak, J. Zarebski, Anal. Bioanal. Chem. 382 (2005) 1691 (https://dx.doi.org/10.1007/s00216-005-3313-2)
S. Karthikeyan, V. K. Gupta, R. Boopathy, A. Titus, G. Sekaran, J. Mol. Liq. 173 (2012) 153 (https://dx.doi.org/10.1016/j.molliq.2012.06.022)
V. K. Gupta, S. Kumar, R. Singh, L. P. Singh, S. K. Shoora, B. Sethi, J. Mol. Liq. 195 (2014) 65 (https://dx.doi.org/10.1016/j.molliq.2014.02.001)
G. Dimeski, T. Badrick, A. S. John, Clin. Chim. Acta 411 (2010) 309 (https://dx.doi.org/10.1016/j.cca.2009.12.005)
N. Mergu, A. K. Singh, V. K. Gupta, Sensors 15 (2015) 9097 (https://doi.org/10.3390/s150409097)
K. Yamada, Y. Nomura, D. Citterio, N. Iwasawa, K. Suzuki, J. Am. Chem. Soc. 127 (2005) 6956 (https://dx.doi.org/10.1021/ja042414o)
Y. M. Poronik, G. Clermont, M. Blanchard-Desce, D. T. Gryko, J. Org. Chem. 78 (2013) 11721 (https://dx.doi.org/10.1021/jo401653t)
T. Gunnlaugsson, M. Nieuwenhuyzen, L. Richard, V. Thoss, J. Chem. Soc. Perkin Trans. 2 (2002) 141 (http://dx.doi.org/10.1039/B106474F)
P. Nandhikonda, M. P. Begaye, M. D. Heagy, Tetrahedron Lett. 50 (2009) 2459 (https://dx.doi.org/10.1016/j.tetlet.2009.02.197)
W. Zhou, J. Ding, J. Liu, Nucleic Acids Res. 44 (2016) 10377 (https://dx.doi.org/10.1093/nar/gkw845)
M. Taki, H. Ogasawara, H. Osaki, A. Fukazawa, Y. Sato, K. Ogasawara, T. Higashiyama, S. Yamaguchi, Chem. Commun. 51 (2015) 11880 (https://dx.doi.org/10.1039/c5cc03547c)
I. Leray, J.-P. Lefevre, J.-F. Delouis, J. Delaire, B. Valeur, Chem. Eur. J. 7 (2001) 4590 (https://doi.org/10.1002/1521-3765(20011105)7:21%3C4590::AID-CHEM4590%3E3.0.CO;2-A)
N. A. Al-Masoudi, N. J. Al-Salihi, Y. A. Marich, J. Fluoresc. 25 (2015) 1847 (https://dx.doi.org/10.1007/s10895-015-1677-z)
J. Al Anshori, D. S. Rahayu, A. T. Hidayat, I. W. Hidayat, A. Zainuddin, Res. J. Chem. Environ. 22 (2018) 91 (https://worldresearchersassociations.com/SpecialIssueAugust2018.aspx)
M. Tasior, D. Kim, S. Singha, M. Krzeszewski, K. H. Ahn, D. T. Gryko, J. Mater. Chem. C 3 (2015) 1421 (https://dx.doi.org/10.1039/C4TC02665A)
Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford, CT, 2016 (https://gaussian.com)
Y. Zhao, D. G. Truhlar, Theor. Chem. Accounts 120 (2008) 215 (https://dx.doi.org/10.1007/s00214-007-0310-x)
J. M. Xiao, L. Feng, L. S. Zhou, H. Z. Gao, Y. L. Zhang, K. W. Yang, Eur. J. Med. Chem. 59 (2013) 150 (https://dx.doi.org/10.1016/j.ejmech.2012.11.019)
J. Piao, J. Lv, X. Zhou, T. Zhao, X. Wu, Spectrochim. Acta, A 128 (2014) 475 (https://dx.doi.org/10.1016/j.saa.2014.03.002)
M. Amirnasr, R. Sadeghi Erami, S. Meghdadi, Sensors Actuators, B 233 (2016) 355 (https://dx.doi.org/10.1016/j.snb.2016.04.077)
S. Goswami, S. Chakraborty, S. Paul, S. Halder, S. Panja, S. K. Mukhopadhyay, Org. Biomol. Chem. 12 (2014) 3037 (https://dx.doi.org/10.1039/C4OB00067F)
X. B. Fu, X. F. Wang, J. N. Chen, D. W. Wu, T. Li, X. C. Shen, J. K. Qin, Molecules 20 (2015) 18565 (https://doi.org/10.3390/molecules201018565)
W. Zhao, L. Pan, W. Bian, J. Wang, Chem. Phys. Chem. 9 (2008) 1593 (https://dx.doi.org/10.1002/cphc.200800131)
X. Liu, J. M. Cole, K. S. Low, J. Phys. Chem., C 117 (2013) 14731 (https://dx.doi.org/10.1021/jp310397z)
R. Wang, F. Zhang, J. Mater. Chem., B 2 (2014) 2422 (https://dx.doi.org/10.1039/C3TB21447H)
T. G. Phan, A. Bullen, Immunol. Cell Biol. 88 (2010) 438 (https://doi.org/10.1038/icb.2009.116)
B. P. Joshi, T. D. Wang, Cancers 2 (2010) 1251 (https://dx.doi.org/10.3390/cancers2021251)
J. Rao, A. Dragulescu-Andrasi, H. Yao, Curr. Opin. Biotechnol. 18 (2007) 17 (https://dx.doi.org/10.1016/j.copbio.2007.01.003)
R. Macgregor, G. Weber, Ann. N.Y. Acad. Sci. 366 (1981) 140 (https://doi.org/10.1111/j.1749-6632.1981.tb20751.x)
A. T. Afaneh, G. Schreckenbach, J. Phys. Chem., A 119 (2015) 8106 (https://dx.doi.org/10.1021/acs.jpca.5b04691)
N. Mergu, M. Kim, Y.-A. Son, Spectrochim. Acta, A 188 (2018) 571 (https://doi.org/10.1016/j.saa.2017.07.047)
T. Keawwangchai, N. Morakot, B. Wanno, J. Mol. Model. 19 (2013) 1435 (https://dx.doi.org/10.1007/s00894-012-1698-3).