The evaluation of chemoselectivity in multicomponent domino Knoevenagel/Diels–Alder reaction: A DFT study Scientific paper
Main Article Content
Abstract
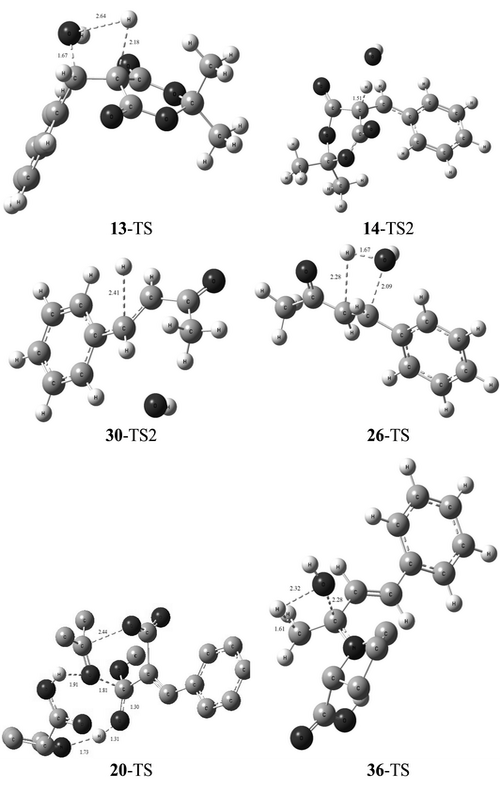
Herein, the chemoselectivity of the multicomponent domino Knoevenagel/Diels–Alder reaction is investigated in terms of theoretical calculations. The structures of reagents, transition states, intermediates and products are optimized at the M062X/6-31+G(d,p) level of theory. The reaction mechanism involves processes of bond rotation, isomerization, asymmetric cycloaddition, acid–base and nucleophile–electrophile competitions, which are studied for the purpose of delivering a clear information of the mechanism in terms of chemoselectivity considerations. Accordingly, the chemoselectivity of the reaction is controlled by the releasing acetone during the decomposition of Meldrum acid in the presence of methanol and l-proline (DG# = 61.45 kcal** mol-1). Comparing calculated results (gas and solvent phase) with the experimental ones showed that using these reagents are the kinetical favourite path for the chemoselective multicomponent cascade Knoevenagel/Diels–Alder reaction to produce the predominant product (>95 %). The results suggest that the creation of cis-spiro cyclohexanone is the predominant chemoselective product under kinetic control of the desired enone.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
L. Reguera, D. G. Rivera, Chem. Rev. 119 (2019) 9836 (https://doi.org/10.1021/acs.chemrev.8b00744)
U. K. Sharma, P. Ranjan, E. V. Van der Eycken, S.-L. You, Chem. Soc. Rev. 49 (2020) 8721 (https://doi.org/10.1039/D0CS00128G)
R. C. Cioc, E. Ruijter, R. V. Orru, Green Chem. 16 (2014) 2958 (https://doi.org/10.1039/C4GC00013G)
H. Pellissier, Adv. Synth. Catal. 358 (2016) 2194 (https://doi.org/10.1002/adsc.201600462)
M. Ashe, Master Thesis, University of Southampton, Faculty of Natural and Environmental Sciences, Southampton, 2016 (http://eprints.soton.ac.uk/id/eprint/397980)
S. M. Xu, L. Wei, C. Shen, L. Xiao, H. Y. Tao, C. J. Wang, Nat. Commun. 10 (2019) 5553 (https://doi.org/10.1038/s41467-019-13529-z)
M. Wang, Z. Shi, Chem. Rev. 120 (2020) 7348 (https://doi.org/10.1021/acs.chemrev.9b00384)
H. A. Younus, M. Al. Rashida, A. Hameed, M. Uroos, U. Salar, S. Rana, K. M. Khan, Expert Opin. Ther. Pat. 31 (2021) 267 (https://doi.org/10.1080/13543776.2021.1858797)
X. Xiao, T. R. Hoye, Nat. Chem. 10 (2018) 838 (https://doi.org/10.1038/s41557-018-0075-y)
M. H. Cao, N. J. Green, S. Z. Xu, Org. Biomol. Chem. 15 (2017) 3105 (https://doi.org/10.1039/C6OB02761J)
X. Ji, C. Zhou, K. Ji, R. E. Aghoghovbia, Z. Pan, V. Chittavong, B. Ke, B. Wang, Angew. Chem. Int. Ed. 55 (2016) 15846 (https://doi.org/10.1002/anie.201608732)
Y. Yamashita, T. Yasukawa, W. J. Yoo, T. Kitanosono, S. Kobayashi, Chem. Soc. Rev. 47 (2018) 4388 (https://doi.org/10.1039/C7CS00824D)
J. Hu, M. Bian, H. Ding, Tetrahedron Lett. 57 (2016) 5519 (https://doi.org/10.1016/j.tetlet.2016.11.007)
J. F. Allochio Filho, B. C. Lemos, A. S. de Souza, S. Pinheiro, S. J. Greco, Tetrahedron 73 (2017) 6977 (https://doi.org/10.1016/j.tet.2017.10.063)
B. L. Oliveira, Z. Guo, G. J. L. Bernardes, Chem. Soc. Rev. 46 (2017) 4895 (https://doi.org/10.1039/C7CS00184C)
P. L. Wang, S. Y. Ding, Z. C. Zhang, Z. P. Wang, W. Wang, J. Am. Chem. Soc. 141 (2019) 18004 (https://doi.org/10.1021/jacs.9b10625)
W. Gati, H. Yamamoto, Acc. Chem. Res. 49 (2016) 1757 (https://doi.org/10.1021/acs.accounts.6b00243)
C. He, J. Hu, Y. Wu, H. Ding, J. Am. Chem. Soc. 139 (2017) 6098 (https://doi.org/10.1021/jacs.7b02746)
E. Sánchez-Larios, J. M. Holmes, C. L. Daschner, M. Gravel, Org. Lett. 12 (2010) 5772 (https://doi.org/10.1021/ol102685u)
J. Wang, H. Li, H. Xie, L. Zu, X. Shen, W. Wang, Angew. Chem. 119 (2007) 9208 (https://doi.org/10.1002/ange.200703163)
B. C. Hong, R. Y. Nimje, A. A. Sadani, J. H. Liao, Org. Lett. 10 (2008) 2345 (https://doi.org/10.1021/ol8005369)
W. Notz, F. Tanaka, C. F. Barbas, Acc. Chem. Res. 37 (2004) 580 (https://doi.org/10.1021/ar0300468)
A. Cordova, C. F. Barbas, Tetrahedron Lett. 44 (2003) 1923 (https://doi.org/10.1016/S0040-4039(03)00019-4)
F. Tanaka, C. F. Barbas III, J. Syn. Org. Chem. Jpn. 63 (2005) 709 (https://doi.org/10.5059/yukigoseikyokaishi.63.709)
S. Mukherjee, J. W. Yang, S. Hoffmann, B. List, Chem. Rev. 107 (2007) 5471 (https://doi.org/10.1021/cr0684016)
N. Campillo, J. A. Paez, P. Goya, Helv. Chim. Acta 86 (2003) 139 (https://doi.org/10.1002/hlca.200390003)
D. B. Ramachary, K. Anebouselvy, N. S. Chowdari, C. F. Barbas, J. Org. Chem. 69 (2004) 5838 (https://doi.org/10.1021/jo049581r)
R. Thayumanavan, B. Dhevalapally, K. Sakthivel, F. Tanaka, C. F. Barbas III, Tetrahedron Lett. 43 (2002) 3817 (https://doi.org/10.1016/S0040-4039(02)00686-X)
N. S. Chowdari, C. F. Barbas, Org. Lett. 7 (2005) 867 (https://doi.org/10.1021/ol047368b)
E. M. Carreira, T. C. Fessard, Chem. Rev. 114 (2014) 8257 (https://doi.org/10.1021/cr500127b)
L. Hong, R. Wang, Adv. Synth. Catal. 355 (2013) 1023 (https://doi.org/10.1002/adsc.201200808)
J. Tellenbröker, D. Kuck, Eur. J. Org. Chem. 2001 (2001) 1483 (https://doi.org/10.1002/1099-0690(200104)2001:8<1483::AID-EJOC1483>3.0.CO;2-U)
A. Boudhar, M. Charpenay, G. Blond, J. Suffert, Angew. Chem. Int. Ed. 52 (2013) 12786 (https://doi.org/10.1002/anie.201304555)
L. Porcelli, D. Stolfa, A. Stefanachi, R. Di Fonte, M. Garofoli, R. Iacobazzi, N. Silvestris, A. Guarini, S. Cellamare, A. Azzariti, Cancer Lett. 445 (2019) 1 (https://doi.org/10.1016/j.canlet.2018.12.013)
D. B. Ramachary, C. F. Barbas III, Chem. Eur. J. 10 (2004) 5323 (https://doi.org/10.1002/chem.200400597)
M. W. Schmidt, K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, J. A. Montomery, J. Comput. Chem. 14 (1993) 1347 (https://doi.org/10.1002/jcc.540141112)
Y. Zhao, D. G. Truhlar, Theor. Chem. Acc. 120 (2008) 215 (https://doi.org/10.1007/s00214-007-0310-x)
A. Castro-Alvarez, H. Carneros, D. Sanchez, J. Vilarrasa, J. Org. Chem. 80 (2015) 11977 (https://doi.org/10.1021/acs.joc.5b01814)
M. Head-Gordon, J. A. Pople, M. J. Frisch, J. Chem. Phys. Lett. 153 (1988) 503 (https://doi.org/10.1016/0009-2614(88)85250-3)
Y. Zhao, N. E. Schultz, D. G. Truhlar, J. Chem. Phys. 123 (2005) 161103 (https://doi.org/10.1063/1.2126975)
S. B. Trickey, Int. J. Quantum Chem. 59 (1996) 259 (https://doi.org/10.1002/qua.560590302)
M. Attarbashi, N. Zabarjad Shiraz, M. Samadizadeh, J. Theor. Comput. Chem. 19 (2020) 2050005 (https://doi.org/10.1142/S0219633620500054)
M. Girod, B. Grammaticos, Nucl. Phys., A 330 (1979) 40 (https://doi.org/10.1016/0375-9474(79)90535-9).