Theoretical calculation of newly synthesized tetrazolopyrimidine derivatives as a potential corrosion inhibitor Scientific paper
Main Article Content
Abstract
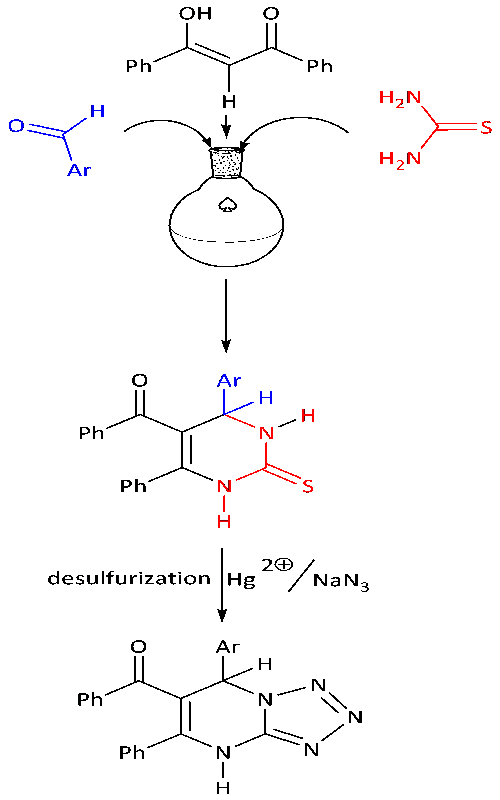
In this work, we wanted to define a general and comprehensive strategy for the synthesis of tetrazolo[1,5-a]pyrimidine derivatives. For this purpose, we obtained new tetrazolo[1,5-a]pyrimidine molecules via the mercury-promoted desulfurization reaction, including hydrolysis, cyclizations, and eliminations. All of the molecules were characterized by FT-IR, 1H-NMR, 13C-
-NMR, and elemental analysis. On the other hand, the potentials of compounds as corrosion inhibitors were calculated at B3LYP/6-31G (d, p) level via density functional theory (DFT).
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Yüzüncü Yil Üniversitesi
Grant numbers FYL-2018- -7181
References
M. Lagrene, B. Mernari, M. Bouanis, M. Traisnel, F. Bentiss, Corrosion Sci. 44 (2002) 573 (https://doi.org/10.1016/S0010-938X(01)00075-0)
A. Yurt, S. Ulutas, H. Dal, Appl. Surf. Sci. 253 (2006) 919 (https://doi.org/10.1016/j.apsusc.2006.01.026)
S. Şafak, B. Duran, A. Yurt, G. Türkoğlu, Corros. Sci. 54 (2012) 25 (https://doi.org/10.1016/j.corsci.2011.09.026)
S. Shahabi, P. Norouzi, M. R. Ganjali, Int. J. Electrochem Sci. 10 (2015) 2646 (https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.668.6592&rep=rep1&type=pdf)
J. Aljourani, M. A. Golozar, K. Raeissi, Mater. Chem. Phys. 121 (2010) 320 (https://doi.org/10.1016/j.matchemphys.2010.01.040)
A. Chetouani, A. Aouniti, B. Hammouti, N. Benchat, T. Benhadda, S. Kertit, Corros. Sci. 45 (2003) 1675 (https://doi.org/10.1016/S0010-938X(03)00018-0)
R. T. Loto, C. A. Loto, A P. I. Popoola, M. Ranyaoa, Int. J. Phys. Sci. 7 (2012) 2697 (https://www.researchgate.net/publication/313580988_Pyrimidine_derivatives_as_environmentally-friendly_corrosion_inhibitors_A_review)
K. Rasheeda, V. D. P. Alva, P. A. Krishnaprasad, S. Samshuddin, Int. J. Corros. Scale Inhib. 7 (2018) 48 (http://ijcsi.pro/papers/pyrimidine-derivatives-as-potential-corrosion-inhibitors-for-steel-in-acid-medium-an-overview/)
H. Dansena, H. J. Dhongade, K. Chandrakar, Asian J. Pharm. Clin. Res. 8 (2015) 171 (https://webcache.googleusercontent.com/search?q=cache:luNzlxY0zn8J:https://innovareacademics.in/journals/index.php/ajpcr/article/download/6283/2710+&cd=2&hl=tr&ct=clnk&gl=tr)
T. P. Selvam, C. R. James, P. V. Dniandev, S. K. Valzita, Research in Pharm. 2 (2012) 01 (https://updatepublishing.com/journal/index.php/rip/article/view/271)
M. Abdallah, E. A. Helal, A. S. Fouda, Corros. Sci. 48 (2006) 1639 (https://doi.org/10.1016/j.corsci.2005.06.020)
D-Q. Zhang, Q-R. Cai, X-M. He, L-X. Gao, G-D. Zhou, Mater. Chem. Phys. 112 (2008) 353 (https://doi.org/10.1016/j.matchemphys.2008.05.060)
M. A. Amin, M. M. Ibrahim, Corros. Sci. 53 (2011) 873 (https://doi.org/10.1016/j.corsci.2010.10.022)
N. A. Wazzan, I. Obot, S. Kaya, J. Mol. Liq. 221 (2016) 579 (https://doi.org/10.1016/j.molliq.2016.06.011)
B. Usman, I. Jimoh, B. A. Umar, Appl. J. Environ. Eng. Sci. 5 (2019) 66 (https://www.researchgate.net/publication/332195552_Theoretical_study_of_2_-3_4-dihydroxyphenyl_chroman-3_5_7-triol_on_corrosion_inhibition_of_mild_steel_in_acidic_medium)
Y. Atalay, F. Yakuphanoglu, M. Sekerci, D. Avcı, A. Başoğlu, Spectrochim. Acta, A 64 (2006) 68 (https://doi.org/10.1016/j.saa.2005.06.038)
E. E. Ebenso, T. Arslan, F. Kandemirli, N. Caner, I. Love, Int. J. Quantum Chem. 110 (2010) 1003 (https://doi.org/10.1002/qua.22249)
F. E. T. Heakal, S. A. Rizk, A. E. Elkholy, J. Mol. Struct. 1152 (2018) 328 (https://doi.org/10.1016/j.molstruc.2017.09.079)
E. Akbas, E. Yildiz, A. Erdogan, J. Serbian Chem. Soc. 85 (2020) 481 (https://doi.org/10.2298/JSC190326081A)
F. Shojaie, N. M. Baghini, Int. J. Ind. Chem. 6 (2015) 297 (https://doi.org/10.1007/s40090-015-0052-x)
Gaussian 09, Revision E.01, Gaussian, Inc., Wallingford, CT, 2009 (http://gaussian.com/g09citation/)
E. Akbas, E. Ergan, E. Sahin, S. Ekin, M. Cakir, Y. Karakus, Phosphorus Sulfur Silicon Relat. Elem. 194 (2019) 796 (https://doi.org/10.1080/10426507.2018.1550489)
E. Akbas, A. Levent, S. Gumus, M. R. Sumer, I. Akyazi, Bull. Korean Chem. Soc. 31 (2010) 3632 (https://doi.org/10.5012/bkcs.2010.31.12.3632)
E. Akbas, S. Celik, E. Ergan, A. Levent, J. Chem. Sci. 131 (2019) 30 (https://doi.org/10.1007/s12039-019-1602-0)
E. Ergan, E. Akbas, A. Levent, E. Sahin, M. Konus, N. Seferoglu, J. Mol. Struct. 1136 (2017) 231 (https://doi.org/10.1016/j.molstruc.2017.02.001)
F. Aslanoglu, E. Akbas, M. Sonmez, B. Anil, Phosphorus Sulfur Silicon Relat. Elem. 182 (2007) 1589 (https://doi.org/10.1080/10426500701263554)
Y. K. Yang, K. J. Yook, J. Tae, J. Am. Chem. Soc. 127 (2005) 16760 (https://doi.org/10.1021/ja054855t)
K. C. Song, J. S. Kim, S. M. Park, K. C. Chung, S. Ahn, S. K. Chang, Org. Lett. 8 (2006) 3413 (https://doi.org/10.1021/ol060788b)
A. D. Becke, J. Chem. Phys. 96 (1992) 2155 (https://doi.org/10.1063/1.462066)
A. D. Becke, J. Chem. Phys. 98 (1993) 1372 (https://doi.org/10.1063/1.464913)
C. Lee, W. Yang, R. G. Parr, Phys. Rev., B 37 (1988) 785 (https://doi.org/10.1103/PhysRevB.37.785)
Y. Karzazi, M. E. A. Belghiti, A. Dafali, B. Hammouti, J. Chem. and Pharm. Res. 6 (2014) 689 (https://www.jocpr.com/abstract/a-theoretical-investigation-on-the-corrosion-inhibition-of-mild-steel-by-piperidine-derivatives-in-hydrochloric-acid-sol-2763.html)
H. Zarrok, A. Zarrouk, R. Salghi, H. Oudda, B. Hammouti, M. Assouag, M. Taleb, M. Ebn Touhami, M. Bouachrine, S. Boukhris, J. Chem. Pharm. Res. 4 (2012) 5056 (https://www.jocpr.com/articles/gravimetric-and-quantum-chemical-studies-of-14acetyl24chlorophenylquinoxalin14hylacetone-as-corrosion-inhibitor-for-carb.pdf)
T. T. Adejumo, N. V. Tzouras, L. P. Zorba, D. Radanovic, A. Pevec, S. Grubišic, D. Mitic, K. K. Andelkovic, G. C. Vougiokalakis, B. Cobeljic, I. Turel, Molecules 25 (2020) 4043 (https://doi.org/10.3390/molecules25184043)
X. Li, S. Deng, H. Fu, T. Li, Electrochim. Acta 54 (2009) 4089 (https://doi.org/10.1016/j.electacta.2009.02.084)
N. Caliskan, E. Akbas, Mater. Corros. 63 (2012) 231 (https://doi.org/10.1002/maco.201005788)
N. Caliskan, E. Akbas, Mater. Chem. Phys. 126 (2011) 983 (https://doi.org/10.1016/j.matchemphys.2010.11.051)
R. Hasanov, M. Sadikoglu, S. Bilgic, Appl. Surf. Sci. 253 (2007) 3913 (https://doi.org/10.1016/j.apsusc.2006.08.025)
F. Bentiss, M. Lagrenée, J. Mater. Environ. Sci. 2 (2011) 13 (https://www.jmaterenvironsci.com/Document/vol2/3-JMES-62-2011-Bentiss2.pdf)
P. Udhayakala, T. V. Rajendiran, S. Gunasekaran, J. Advanced Sci. Res. 3 (2012) 71 (http://eds.b.ebscohost.com/eds/pdfviewer/pdfviewer?vid=1&sid=85d1e953-13bc-4e39-94f0-6c41acdeacb8%40sessionmgr102).