Terpenoids in four Inula species from Bulgaria Scientific paper
Main Article Content
Abstract
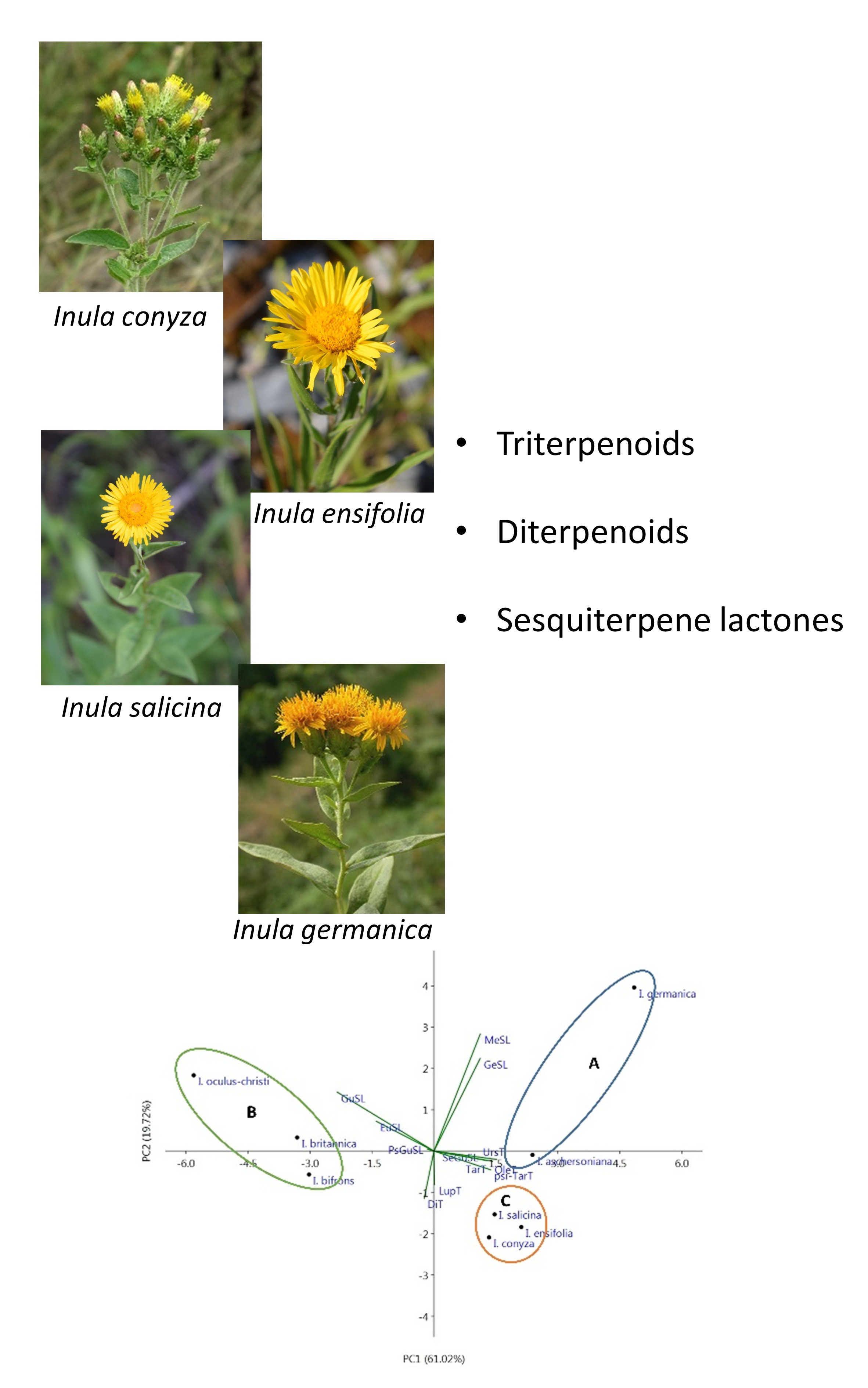
Phytochemical study of the chloroform extract of the aerial parts of Inula germanica L., I. ensifolia L., I. conyza (Griess.) DC. and I. salicina L. led to the identification of 33 terpenoids. β- and α-amyrin, lupeol, taraxasterol, ψ-taraxasterol and their 3-O-acetates and 3-O-palmitates were identified by GC/MS. In addition, the structures of 3-O-palmitates of mainaladiol, arnidiol, faradiol and 16-hydroxylupeol were confirmed by NMR. ent-Kaur-16-en-19-oic acid and its 15α-(3-methylpentanoyloxy) and 15α-(3-methylbutanoyloxy) derivatives were isolated from I. conyza. Ten closely related sesquiterpene lactones (germacranolides and melampolides) were found in I. germanica and their structural identification was performed by spectral analyses. I. ensifolia and I. salicina were free of sesquiterpene lactones and diterpenoids. All triterpenoids and diterpenoids, grazielia acid, desacetylovatifolin and 8-(2-methylbutanoyloxy)-1(10),4,11(13)-germacrutrien-6,12-olide-14-oic acid are described for the first time in the studied species. The principal component analysis was used to find a relationship between thе investigated up to now Inula species, growing in Bulgaria.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
G. Beck, “Inulae Europae”: Die Europäischen Inula-Arten, Dankschr. Königl. Akad. Wiss. Mat.-Nat., Nabu Press, Charleston, SC, 2011
A. A. Anderberg, Plant Syst. Evol. 176 (1991) 75 (https://dx.doi.org/10.1007/BF00937947)
A. M. L. Seca, A. Grigore, D. C. G. A. Pinto, A. M. S. Silva, J. Ethnopharmacol. 154 (2014) 286 (https://dx.doi.org/10.1016/j.jep.2014.04.010)
A. M. L. Seca, D. C. G. A. Pinto, A. M. S. Silva, Chem. Biodivers. 12 (2015) 859 (https://dx.doi.org/10.1002/cbdv.201400080)
Y.-M. Zhao, M.-L. Zhang, Q.-W. Shi, H. Kiyota, Chem. Biodivers. 3 (2006) 371 (https://dx.doi.org/10.1002/CBDV.200690041)
A. L. Khan, J. Hussain, M. Hamayun, S. A. Gilani, S. Ahmad, G. Rehman, Y. H. Kim, S. M. Kang, I. J. Lee, Molecules 15 (2010) 1562 (https://dx.doi.org/10.3390/molecules15031562)
F. C. Seaman, Bot. Rev. 1982 482 48 (1982) 121 (https://dx.doi.org/10.1007/BF02919190)
A. Trendafilova, V. Ivanova, M. Todorova, I. Aneva, Phytochem. Lett. 21 (2017) 221 (https://dx.doi.org/10.1016/J.PHYTOL.2017.07.008)
A. Trendafilova, M. Todorova, I. Aneva, Comptes Rendus Acad. Bulg. Des. Sci. 71 (2018) 341 (https://dx.doi.org/10.7546/CRABS.2018.03.05)
V. Ivanova, A. Trendafilova, M. Todorova, K. Danova, D. Dimitrov, Nat. Prod. Commun. 12 (2017) 153 (https://dx.doi.org/10.1177/1934578x1701200201).
A. Trendafilova, M. Todorova, V. Genova, P. Shestakova, D. Dimitrov, M. Jadranin, S. Milosavljevic, Nat. Prod. Commun. 9 (2014) 1123 (https://dx.doi.org/10.1177/1934578x1400900814)
V. Ivanova, M. Todorova, I. Aneva, P. Nedialkov, A. Trendafilova, Biochem. Syst. Ecol. 93 (2020) 104141 (https://dx.doi.org/10.1016/J.BSE.2020.104141)
H. Budzikiewicz, J. M. Wilson, C. Djerassi, J. Am. Chem. Soc. 85 (1963) 3688 (https://dx.doi.org/10.1021/ja00905a036)
T. Kundakovic, N. Fokialakis, P. Magiatis, N. Kovacevic, I. Chinou, Chem. Pharm. Bull. 52 (2004) 1462 (https://dx.doi.org/10.1248/cpb.52.1462)
C. Y. Ragassa, F. Tiu, J. A. Rideoout, ACGC Chem. Res. Comm. 18 (2005) 11
S. Öksüz, G. Topçu, Phytochemistry 31 (1992) 195 (https://dx.doi.org/10.1016/0031-9422(91)83034-I)
T. Kikuchi, A. Tanaka, M. Uriuda, T. Yamada, R. Tanaka, Molecules 21 (2016) 1121 (https://dx.doi.org/10.3390/molecules21091121)
A. Trendafilova, M. Todorova, N. Kutova, M. Guncheva, Nat. Prod. Commun. 13 (2018) 1017–1020 (https://dx.doi.org/10.1177/1934578X1801300823)
C. Nicolaus, S. Junghanns, R. Murillo, I. Merfort, Planta Med. 80 (2014) P2B63 (https://dx.doi.org/10.1055/S-0034-1394940)
N. Ohno, T. J. Mabry, V. Zabelt, W. H. Watson, Phytochemistry 18 (1979) 1687 (https://dx.doi.org/10.1016/0031-9422(79)80184-3)
M. Velikova, V. Bankova, I. Tsvetkova, A. Kujumgiev, M. C. Marcucci, Fitoterapia 71 (2000) 693 (https://dx.doi.org/10.1016/S0367-326X(00)00213-6)
L. Ding, K. Wang, H. Wang, W. Tu, Z. Deng, Y. Zhou, L. Song, J. Chinese Med. Mater. 39 (2016) 1296
J. J. Qin, J. X. Zhu, W. D. Zhang, Y. Zhu, J. J. Fu, X. H. Liu, H. Z. Jin, Arch. Pharm. Res. 32 (2009) 1369 (https://dx.doi.org/10.1007/s12272-009-2004-5)
Z. P. Yu, J. H. Yu, J. S. Zhang, S. J. Yu, H. Zhang, Tetrahedron 75 (2019) 130732 (https://dx.doi.org/10.1016/j.tet.2019.130732)
F. Bohlmann, C. Zdero, R. M. King, H. Robinson, Phytochemistry 20 (1981) 1069 (https://dx.doi.org/10.1016/0031-9422(81)83029-4)
A. Kijjoa, M. M. S. M. Bastos, T. E. Gedris, W. Herz, Phytochemistry 32 (1993) 383 (https://dx.doi.org/10.1016/S0031-9422(00)94999-9)
M. Hoeneisen, M. Sicva, F. Bohlmann, Phytochemistry 19 (1980) 2765 (https://dx.doi.org/10.1016/S0031-9422(00)83964-3)
C. L. Cespedes, M. Hoeneisen, M. Bittner, J. Becerra, M. Silva, J. Agric. Food Chem. 49 (2001) 4243 (https://dx.doi.org/10.1021/jf010351c)
O. A. Konovalova, K. S. Rybalko, V. I. Sheichenko, Chem. Nat. Compd. 1976 105 10 (1974) 591 (https://dx.doi.org/10.1007/BF00567848)
K. Schorr, I. Merfort, F. B. Da Costa, Nat. Prod. Commun. 2 (2019) 367 (https://dx.doi.org/10.1177/1934578X0700200404)
F. Bohlmann, R. N. Baruah, J. Jakupovic, Planta Med. 51 (1985) 261 (https://dx.doi. org/10.1055/S-2007-969474)
F. Bohlmann, P. K. Mahanta, J. Jakupovic, R. C. Rastogi, A. A. Natu, Phytochemistry 17 (1978) 1165 (https://dx.doi.org/10.1016/S0031-9422(00)94308-5).