Correlation of the solubility of solid hydrocarbons in supercritical CO2 using different equations of state and mixing rules Scientific paper
Main Article Content
Abstract
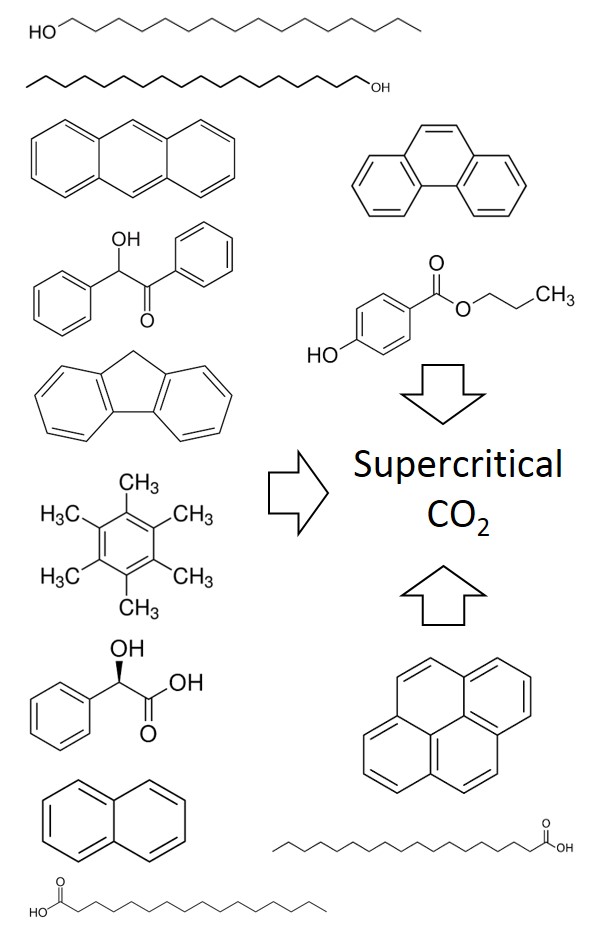
The supercritical extraction process is a technique that has increasingly been applied in various industries in recent years. Solubility determination in the supercritical region is the key feature for this process. However, high expenses and time consuming experiments for this task obligates the need for process modeling. In this study, a thermodynamic model is proposed to correlate the solubility of solid hydrocarbons, namely, 1-hexadecanol, 1-octadecanol, anthracene, benzoin, fluorene, hexamethylbenzene, mandelic acid, naphthalene, palmitic acid, phenanthrene, propyl 4-hydroxybenzoate, pyrene and stearic acid in supercritical conditions, using Peng–Robinson (PR) and Soave–Redlich–Kwong (SRK) equations of state with one-parameter van der Waals (vdW1) and two-parameters (vdW2) and covolume dependent (CVD) mixing rules. For the above combination of equations of state and mixing rules, binary interaction parameters were determined, utilizing the differential evolution optimization strategy. The validity of the model was assessed by comparing the experimental solubility data with the results obtained from thermodynamic model based on average absolute relative deviation (AARD). An empirical correlation was proposed for the correlation of the solid solubilities in supercritical CO2. For each compound, the constants of this equation were obtained in such a manner to correlate the solubility at different temperatures and pressures.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
R. D. Smith, J. P. Blitz, J. L. Fulton, in Supercritical Fluid Science, Technology, Vol. 406, K. P. Johnston, J. M. L. Penninger, Eds., ACS Publications, Washington DC, 1989, pp. 165
A. Chafer, T. Fornari, A. Berna, R. P. Stateva, J. Supercrit. Fluids 32 (2004) 89 (https://doi.org/10.1016/j.supflu.2004.02.005)
H. Yang, C. Zhong, J. Supercrit. Fluids 33 (2005) 99 (https://doi.org/10.1016/j.supflu.2004.05.008)
A. J. Schultz, K. R. S. Shaul, S. Yang, D. A. Kofke, J. Supercrit. Fluids 55 (2010) 479 (https://doi.org/10.1016/j.supflu.2010.10.042)
A. Tarasova, F. Burden, J. Gasteiger, D. A. Winkler, J. Mol. Graphics Modell. 28 (2010) 593 (https://doi.org/10.1016/j.jmgm.2009.12.004)
A. Z. Hezave, M. H. Khademi, F. Esmaeilzadeh, Fluid Phase Equilib. 313 (2012) 140 (https://doi.org/10.1016/j.fluid.2011.09.031)
C.-Y. Huang, L.-S. Lee, C.-S. Su, J. Taiwan Inst. Chem. Eng. 44 (2013) 349 (https://doi.org/10.1016/j.jtice.2012.12.004)
C.-S. Su, J. Supercrit. Fluids 81 (2013) 79 (https://doi.org/10.1016/j.supflu.2013.05.001)
S. A. Shojaee, H. Rajaei, A. Z. Hezave, M. Lashkarbolooki, F. Esmaeilzadeh, J. Supercrit. Fluids 81 (2013) 42 (https://doi.org/10.1016/j.supflu.2013.04.013)
S.-H. Cheng, F.-C. Yang, Y.-H. Yang, C.-C. Hu, W.-T. Chang, J. Taiwan Inst. Chem. Eng. 44 (2013) 19 (https://doi.org/10.1016/j.jtice.2012.09.001)
J. Sakabe, H. Uchida, Y. Shimoyama, Chem. Eng. Res. Des. 92 (2014) 2970 (https://doi.org/10.1016/j.cherd.2014.08.003)
M. A. Khansary, F. Amiri, A. Hosseini, A. H. Sani, H. Shahbeig, Chem. Eng. Res. Des. 93 (2015) 355 (https://doi.org/10.1016/j.cherd.2014.05.004)
H. Li, D. Jia, R. Liu, B. Shen, Fluid Phase Equilib. 385 (2015) 10 (https://doi.org/10.1016/j.fluid.2014.10.036)
K. P. Johnston, C. A. Eckert, AlChE J. 27 (1981) 773 ( https://doi.org/10.1002/aic.690270511)
A. Kramer, G. Thodos, J. Chem. Eng. Data 33 (1988) 230 (https://doi.org/10.1021/je00053a002)
A. Kramer, G. Thodos, J. Chem. Eng. Data 34 (1989) 184 (https://doi.org/10.1021/je00056a011)
E. Kosal, G. D. Holder, J. Chem. Eng. Data 32 (1987) 148 (https://doi.org/10.1021/je00048a005)
J. M. Dobbs, K. P. Johnston, Ind. Eng. Chem. Res. 26 (1987) 1476 (https://doi.org/10.1021/ie00067a035)
J. Kwiatkowski, Z. Lisicki, W. Majewski, Ber. Bunsen. Phys. Chem. 88 (1984) 865 (https://doi.org/10.1002/bbpc.19840880919)
K. D. Bartle, A. A. Clifford, S. A. Jafar, J. Chem. Eng. Data 35 (1990) 355 (https://doi.org/10.1021/je00061a037)
M. McHugh, M. E. Paulaitis, J. Chem. Eng. Data 25 (1980) 326 (https://doi.org/10.1021/je60087a018)
M. Mukhopadhyay, Natural extracts using supercritical carbon dioxide, CRC press, Cleveland, OH, 2000
G. M. Kontogeorgis, G. K. Folas, Thermodynamic models for industrial applications: from classical, advanced mixing rules to association theories, John Wiley & Sons, Hoboken, NJ, 2009
I. Polishuk, I. Kapry, M. Madar, Chem. Eng. Commun. 196 (2008) 448 (https://doi.org/10.1080/00986440802483970)
R. C. Reid, J. M. Prausnitz, B. E. Poling, The properties of gases, liquids, McGraw Hill Book Co., New York, 1987
K. P. Johnston, D. H. Ziger, C. A. Eckert, Ind. Eng. Chem. Fundam. 21 (1982) 191 (https://doi.org/10.1021/i100007a001).