Optimization of the reaction conditions for the synthesis of 2,3,5-trimethylpyridine from 3-amino-2-methylpropenal and methylethylketone Short Communication
Main Article Content
Abstract
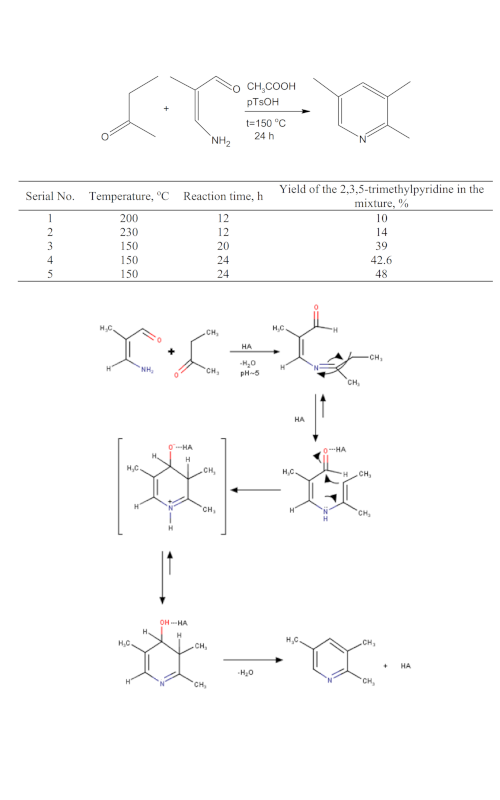
The influence of temperature, reaction time, and type of the catalyst on the yield of the 2,3,5-trimethylpyridine (collidine) from 3-amino-2-methylpropenal and methylethylketone was investigated. 3-Amino-2-methylpropenal was synthesized from 3-ethoxy-2-methylacrolein previously synthesized from methylmalondialdehyde tetraethyl acetal, obtained from triethyl orthoformate and propenyl ether. The optimal conditions for the investigated synthesis were temperature of 150 °C, reaction time 24 h, and the CH3COOH/pTsOH catalyst. This synthesis is the first successful attempt to synthesize 2,3,5-trimethylpyridine in an acid medium.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200124
References
J. C. Sih (Upjohn Co.), US4575554 (1986)
A. Eckert, S. Loria, Monatsh. Chem. 38 (1917) 225 (https://doi.org/10.1007/BF01524213)
M. P. Oparina, Ber. Dtsch. Chem. Ges., B 64 (1931) 562 (https://doi.org/10.1002/cber.19310640311)
T. Eguchi, Bull. Chem. Soc. Jpn. 3 (1928) 235 (https://doi.org/10.1246/bcsj.3.235)
J. Herzenberg, G. Boccato, Chim. Ind. (Paris) 80 (1958) 248
K. Tsuda, N. Ikekawa, H. Mishima, A. Iino, T. Morishige, Pharm. Bull. 1 (1953) 122 (https://doi.org/10.1248/cpb1953.1.122)
G. Errera, Ber. Dtsch. Chem. Ges. 34 (1901) 3691 (https://doi.org/10.1002/cber.19010340367)
E. Breitmaier, S. Gassenmann, Chem. Ber. 104 (1971) 665 (https://doi.org/10.1002/cber.19711040234)
Y. Wakatsuki, H. Yamazaki, Synthesis 1976 (1976) 26 (https://doi.org/10.1055/s-1976-23943)
N. Srinivas, V. Radha Rani, S. J. Kulkarni, K. V. Raghavan, J. Catal. 208 (2002) 332 (https://doi.org/10.1006/jcat.2002.3538)
D.-S. Kim, J.-W. Park, C.-H. Jun, Chem. Commun. 48 (2012) 11334 (https://doi.org/10.1039/C2CC36699A)
J. M. Neely, T. Rovis, J. Am. Chem. Soc. 136 (2014) 2735 (https://doi.org/10.1021/ja412444d)
V. T. Klimko, T.V. Protopopova, N.V. Smirnova, A.P. Skoldinov, Zh. Obshch. Khim. 32 (1962) 2961
V. T. Klimko, T.V. Protopopova, A. P. Skoldinov, SU136351A1 (1960)
J. Vymetal, Z. Hejda, Collect. Czech. Chem. Commun. 43 (1978) 3024 (https://doi.org/10.1135/cccc19783024).