Synthesis of methyl 3,4-anhydro-6-bromo-2-O-tert-butyldimethylsilyl-6-deoxy-α-D-allopyranoside from α-D-glucose Scientific paper
Main Article Content
Abstract
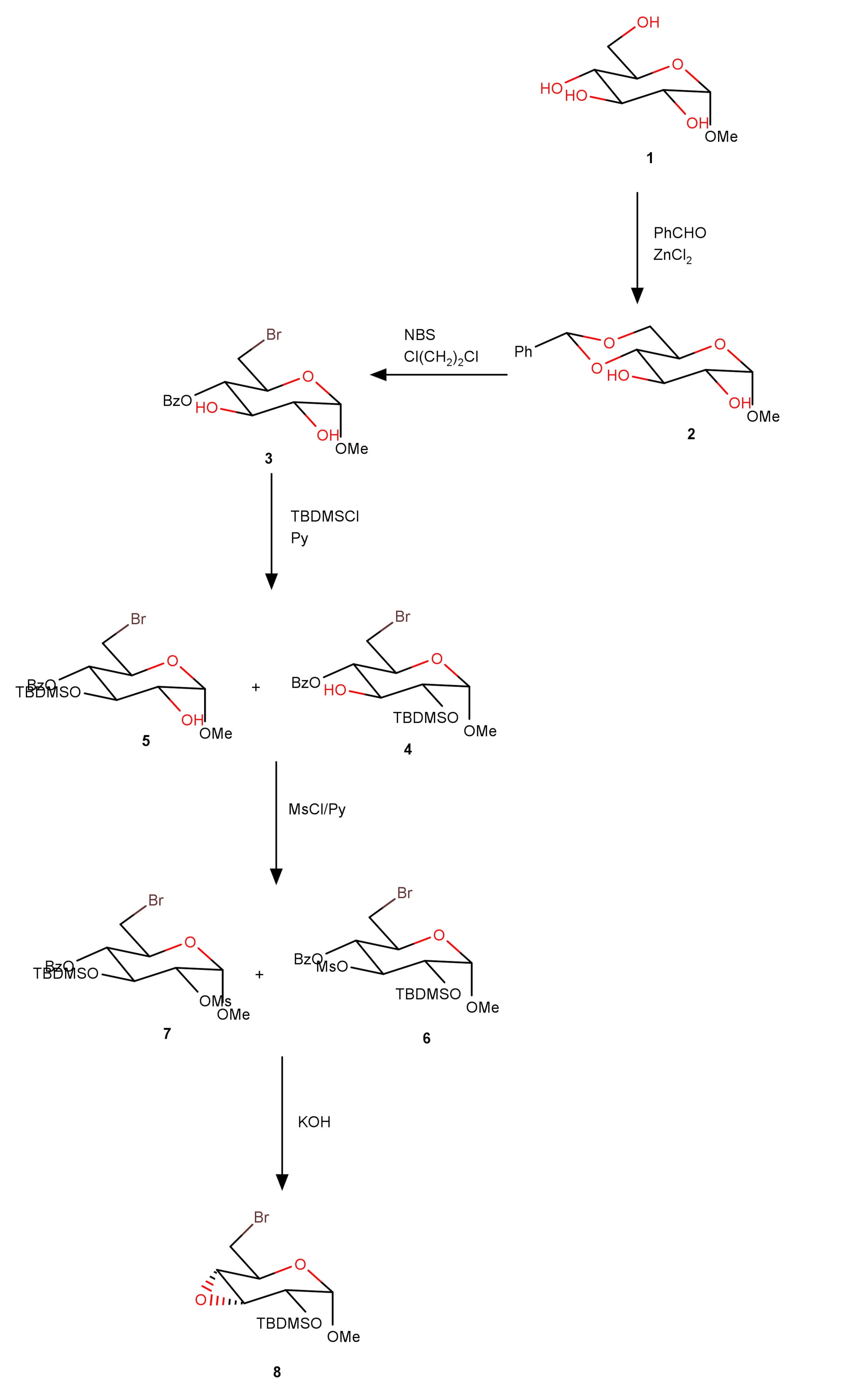
Some of simple carbohydrates and their derivatives are used for the clinical treatment of various diseases. Epoxide derivatives, which can be obtained by the intramolecular elimination of water from two vicinal hydroxyl groups, are stable, but sufficiently reactive compounds very often used as intermediaries in various syntheses. Synthesis of epoxide derivative, methyl 3,4-anhydro-6-bromo-2-O-tert-butyldimethylsilyl-6-deoxy-α-d-allopyranoside from α-d-glucose was achieved in high yields in the minimal number of synthetic steps. Anhydrous glucose was used as a starting material which was transformed into methyl α-d-glucopyranoside using dry, gaseous hydrogen chloride. Thus obtained derivative was treated with benzaldehyde in the presence of zinc chloride as Lewis acid giving methyl (R)-4,6-O-benzylidene-α-d-glucopyranoside. The obtained compound was treated with N-bromosuccinimide (NBS) in dichloromethane in the presence of barium carbonate giving methyl 4-O-benzoyl-6-bromo-6-deoxy-α-d-glucopyranoside. In the next step, the obtained compound was treated with tert-butyldimethylsilyl chloride (TBDMSCl) in pyridine, and methyl 4-O-benzoyl-6-bromo-2-O-tert-butyldimethylsilyl-6-deoxy-α-d-glucopyranoside was further mesylated, and the obtained methyl 4-O-benzoyl-6-bromo-2-O-tert-butyldimethylsilyl-6-deoxy-3-O-mesyl-α-d-glucopyranoside was treated at the end with KOH to give methyl 3,4-anhydro-6-bromo-2-O-tert-butyldimethylsilyl-6-deoxy-α-d-allopyranoside (yield 78 %).
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. A. Barkes, E. J. Bourne, Adv. Carbohydr. Chem. 7 (1952) 137 (https://doi.org/10.1016/S0096-5332(08)60084-3)
A. N. De Belder, Adv. Carbohydr. Chem. 20 (1965) 219 (https://doi.org/10.1016/S0096-5332(08)60300-8)
N. Mishra, V. K. Tiwari, R. R. Schmidt, in Synthesis and Application, V. Kumar Tiwari, Ed., Elsevier Inc., Amsterdam, 2020, pp. 1–69 (https://doi.org/10.1016/B978-0-12-816675-8.00001-4)
C. Piantadosi, C. E. Anderson, E. A. Brecht, C. L. Yarbro, J. Am. Chem. Soc. 80 (1958) 6613 (https://doi.org/10.1021/ja01557a040)
S. Penjarla, S. R. Prasad, D. S. Reddy, S. Banerjee, S. Penta, Y. S. Sanghvi, Nucleosides Nucleotides Nucleic Acids 37 (2018) 232 (https://doi.org/10.1080/15257770.2018.1460480)
J. S. Brimacombe, A. B. Foster, B. D. Jones, J. J. Willard, J. Chem. Soc., C (1967) 2404 (https://doi.org/10.1039/J39670002404)
E. J. Corey, A.Venkateswarlu, J. Am. Chem. Soc. 94 (1972) 6190 (https://doi.org/10.1021/ja00772a043)
V. H. Jadhav, S. B. Lee, H.-J. Jeong, S. T. Lim, M.-H. Sohn, D.W. Kim, Tetrahedron Lett. 53 (2012) 2051 (https://doi.org/10.1016/j.tetlet.2012.02.016)
K. K. Ogilvie, D. J. Iwacha, Tetrahedron Lett. 14 (1973) 317 (https://doi.org/10.1016/S0040-4039(01)95650-3)
T. Halmos, R. Montserret, J. Filippi, K. Antonakis, Carbohydr. Res. 170 (1987) 57 (https://doi.org/10.1016/0008-6215(87)85005-X)
A. Das, A. Bhaumik, T. Pathak, Carbohydr. Res. 487 (2020) 107870 (https://doi.org/10.1016/j.carres.2019.107870)
Dj. Glišin, O. Jovanović, G. Stojanović, Zbornik radova Filozofskog fakulteta u Nišu, serija fizika i hemija 1 (1988) 137 (UDK 542.91:547.455.6) (in Serbian)
H. S. El Khadem, Carbohydrates in Encyclopedia of Physical Science and Technology (Third ed.), Academic Press, Cambridge, MA, 2003, pp. 369–416 (https://doi.org/10.1016/B0-12-227410-5/00080-6)
O. Achmatowicz, R. Bielski, Carbohydr. Res. 55 (1977) 165 (https://doi.org/10.1016/S0008-6215(00)84452-3)
D. M. Hall, Carbohydr. Res. 86 (1980) 158 (https://doi.org/10.1016/S0008-6215(00)84593-0)
M. E. Evans, Carbohydr. Res. 21 (1972) 473 (https://doi.org/10.1016/S0008-6215(00)84931-9)
J. W. Van Cleve, Carbohydr. Res. 17 (1971) 461 (https://doi.org/10.1016/S0008-6215(00)82557-4)
S. Hanessian, in General Carbohydrate Method, R. L. Whistler, J. N. BeMiller, Eds., Academic Press, Cambridge, MA, 1972, pp. 183–189 (https://doi.org/10.1016/B978-0-12-746206-6.50035-7)
S. Hanessian, N. R. Plessas, J. Org. Chem. 34 (1969) 1035 (https://doi.org/10.1021/jo01256a059)
A. M. Castillo, L. Patiny, J. Wist, J. Magn. Reson. 209 (2011) 123 (https://doi.org/10.1016/j.jmr.2010.12.008)
M. M. Manson, Br. J. Ind. Med. 37 (1980) 317 (https://doi.org/10.1136/oem.37.4.317)
B. Kaur, P. Singh, Bioorg. Chem. 125 (2022) 105862 (https://doi.org/10.1016/j.bioorg.2022.105862).