A study towards the synthesis of (–)-atrop-abyssomicin C core Scientific paper
Main Article Content
Abstract
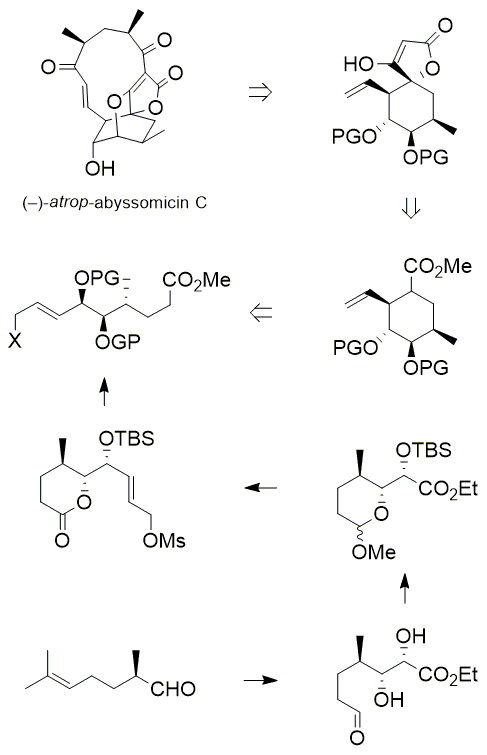
An attempt to synthesize the cyclohexane core of antibiotic abyssomicin C is described. The initial, protecting group-free approach (relying on internal protection) failed and had to be modified, in order to allow for efficient deprotection of the acid-sensitive cyclization precursor in the penultimate synthetic step. Thus, a pyranoside structural unit was used as a latent lactone/ester functionality, which was deprotected via thioacetalization/hydrolysis/oxidation sequence, to give the d-valerolactone-type cyclization precursor. Unfortunately, the key cyclization reaction was not feasible, even after structural modification of the cyclization precursor. Reluctance towards cyclization turned out to be a general property of (at least some) Delta7-unsaturated esters, which required the development of a new strategy for this type of transformation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
a) B. Bister, D. Bischoff, M. Ströbele, J. Riedlinger, A. Reicke, F. Wolter, A. T. Bull, H. Zähner, H.P. Fiedler, R. D. Süssmuth, Angew. Chem. Int. Ed. 43 (2004) 2574 (https://doi.org/10.1002/anie.200353160); b) C. Sadaka, E. Ellsworth,P. R. Hansen, R. Ewin, P. Damborg, J. L. Watts, Molecules 23 (2018) 1371 (https://doi.org/10.3390/molecules23061371)
a) R. M. Hanson, K. B. Sharpless, J. Org. Chem. 51 (1986) 1922 (https://doi.org/10.1021/jo00360a058); b) Y. Gao, J. M. Klunder, R. M. Hanson, H. Masamune, S. Y. Ko, K. B. Sharpless, J. Am. Chem. Soc. 109 (1987) 5765 (https://doi.org/10.1021/ja00253a032)
D. F. Taber, J.B. Houze, J. Org. Chem. 59 (1994) 4004 (https://doi.org/10.1021/jo00093a037)
D. F. Taber, K. K. You, J. Am Chem. Soc. 117 (1995) 5761 (https://doi.org/10.1021/ja00126a015)
J. Chun, H. S. Byun, R. Bittman, J. Org. Chem. 68 (2003) 348 (https://doi.org/10.1021/jo026240+)
A. B. Smith III, M. Fukui, H. A. Vaccaro, J. R. Empfield, J. Am. Chem. Soc. 113 (1991) 2071 (https://doi.org/10.1021/ja00006a029)
D. E. Plaumann, B. J. Fitzsimmons, B. M. Ritchie, and B. Fraser-Reid, J. Org. Chem. 47 (1982) 941 (https://doi.org/10.1021/jo00345a008)
V. G. Saraswathy, S. Sankararaman, J. Org. Chem. 59 (1994) 4665 (https://doi.org/10.1021/jo00095a049)
V. Kumar, S. Dev, Tetrahedron Lett. 24 (1983) 1289 (https://doi.org/10.1016/S0040-4039(00)81637-8)
a) R. Bernardi, D. Ghiringhelli, J. Org. Chem. 52 (1987) 5021 (https://doi.org/10.1021/jo00231a033); b) D. T. Hung, J. B. Nerenberg, S. L. Schreiber, J. Am. Chem. Soc. 118 (1996) 11054 (https://doi.org/10.1021/ja961374o)
R. K. Crossland, K. L. Servis, J. Org. Chem. 35 (1970) 3195 (https://doi.org/10.1021/jo00834a087)
J. P. Ferezou, M. Julia, Y. Li, L. W. Liu, A. Pancrazi, Synlett 12 (1990) 766 (https://doi.org/10.1055/s-1990-21245)
a) F. Bihelovic, R. N. Saicic, Angew. Chem. Int. Ed. 51 (2012) 5687 (https://doi.org/10.1002/anie.201108223); b) F. Bihelovic, I. Karadzic, R. Matovic, R. N. Saicic, Org. Biomol. Chem. 11 (2013) 5413 (https://doi.org/10.1039/C3OB40692J)
a) F. Bihelovic, R. Matovic, B. Vulovic, R. N. Saicic, Org. Lett. 9 (2007) 5063 (https://doi.org/10.1021/ol7023554); b) B. Vulovic, F. Bihelovic, R. Matovic, R. N.Saicic, Tetrahedron 65 (2009) 10485 (https://doi.org/10.1016/j.tet.2009.10.006)
a) E. J. Corey, H. Cho, C. Rücker, D. H. Hua, Tetrahedron Lett. 22 (1981) 3455, (https://doi.org/10.1016/S0040-4039(01)81930-4) b) D. M. Jones, B. Nilsson, M. Szelke, J. Org. Chem. 58 (1993) 2286 (https://doi.org/10.1021/jo00060a052)
G. Stork, R. Mook Jr., S. A. Biller, S. D. Rychnovsky, J. Am. Chem. Soc. 105 (1983) 3741 (https://doi.org/10.1021/ja00349a082)
D. E. Plaumann, B. J. Fitzsimmons, B. M. Ritchie, B. Fraser-Reid, J. Org. Chem. 47 (1982) 941 (https://doi.org/10.1021/jo00345a008)
S. D. Meyer, S. L. Schreiber, J. Org. Chem. 59 (1994) 7549 (https://doi.org/10.1021/jo00103a067).