Synthetic study on the angular triquinanes Scientific paper
Main Article Content
Abstract
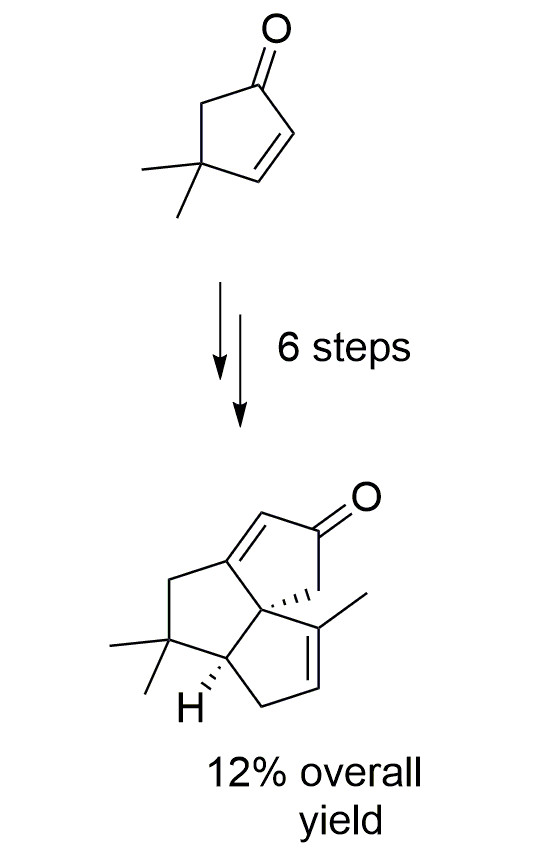
The synthesis of an angular triquinane, which could serve as a suitable platform for the synthesis of several natural products (panaginsene, silphinene, senoxydene) is described. The synthesis is based on two consecutive cyclopentene annulations, where alkenes were used as latent carbonyl functionalities (via Wacker reaction), and cyclopentenone annulation was effected by aldol condensation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers Contract number: 451-03-47/2023-01/200168
References
For review articles on triquinane synthesis, see: a) G. Mehta, A. Srikrishna, Chem. Rev. 97 (1997) 671 (https://doi.org/10.1021/cr9403650); b) L. A. Paquette, A. M. Doherty, Polyquinane Chemistry, 1st ed., Springer, Heidelberg, 1987 (https://doi.org/10.1007/978-3-642-72598-2); c) L. A. Paquette, De Gruyter, Top. Curr. Chem. 119 (1984) 1 (https://doi.org/doi:10.1515/9783112539286); d L. A. Paquette, Top. Curr. Chem. 79 (1979) 41 (https://doi.org/10.1007/BFb0048476); e) M. B. Chanon R; Baralotto, C; Julliard, M; Hendrickson, J B, Synthesis (Stuttg). 11 (1998) 1559 (https://doi.org/10.1055/s-1998-2191); For a recent review article on the synthesis of tetraquinanes, see: f) S. Kotha, A. Fatma, Asian J. Org. Chem. 11 (2022)e202100595 (https://doi.org/10.1002/ajoc.202100595)
a) A. Groweiss, W. Fenical, H. Cun-heng, J. Clardy, W. Zhongde, Y. Zhongnian, L. Kanghou, Tetrahedron Lett. 26 (1985) 2379–2382 (https://doi.org/10.1016/S0040-4039(00)94832-9); b) See also: V. Dragojlovic, Molecules 5 (2000) 674 (https://doi.org/10.3390/50400674)
B.-H. Chen, K.-F. Jiao, Q.-E. Ji, H.-Q. Song, J. Mol. Struct. THEOCHEM 188 (1989) 167 (https://doi.org/10.1016/0166-1280(89)85035-3)
X. Tan, H. Ye, L. Zeng, Z. Cui, S. He, Zhongguo Haiyang Yaowu 9 (1990) 11 (Chemical Abstracts 1991, 115: 35564g) [C.A 115/1991 35564g]
D. E. Cane, Sesquiterpene Biosynthesis: Cyclization Mechanisms in Compr. Nat. Prod. Chem., Sir D. Barton, K. Nakanishi, O. Meth-Cohn, Eds., Pergamon Press, Oxford, 1999, pp. 155–200 (https://doi.org/10.1016/B978-0-08-091283-7.00039-4)
a) I. R. George, M. López-Tena, A. P. Sundin, D. Strand, Org. Lett. 23 (2021) 3536 (https://doi.org/10.1021/acs.orglett.1c00955); b) T. Kobayashi, Y. Teshigahara, M. Sakakibara, K. Murakami, Y. Kawamoto, H. Ito, Org. Lett. 25 (2023) 4510 (https://doi.org/10.1021/acs.orglett.3c01530)
S. H. Shim, J. B. Gloer, D. T. Wicklow, J. Nat. Prod. 69 (2006) 1601 (https://doi.org/10.1021/np060327z)
R. Richter, S. Basar, A. Koch, W. A. König, Phytochemistry 66 (2005) 2708 (https://doi.org/10.1016/j.phytochem.2005.09.012)
S. Geum, H.-Y. Lee, Org. Lett. 16 (2014) 2466 (https://doi.org/10.1021/ol500849m)
T. Tsunoda, M. Kodama, S. Itô, Tetrahedron Lett. 24 (1983) 83 (https://doi.org/10.1016/S0040-4039(00)81333-7)
L. A. Paquette, A. Leone-Bay, J. Am. Chem. Soc. 105 (1983) 7352 (https://doi.org/10.1021/ja00363a024)
D. D. Sternbach, J. W. Hughes, D. F. Burdi, B. A. Banks, J. Am. Chem. Soc. 107 (1985) 2149 (https://doi.org/10.1021/ja00293a053)
P. A. Wender, R. J. Ternansky, Tetrahedron Lett. 26 (1985), 2625 (https://doi.org/10.1016/S0040-4039(00)98120-6)
a) M. T. Crimmins, S. W. Mascarella, J. Am. Chem. Soc. 108 (1986) 3435 (https://doi.org/10.1021/ja00272a044); b) M. T. Crimmins, S. W. Mascarella, Tetrahedron Lett. 28 (1987) 5063 (https://doi.org/10.1016/S0040-4039(00)95590-4)
Y. K. Rao, M. Nagarajan, Tetrahedron Lett. 29 (1988) 107 (https://doi.org/10.1016/0040-4039(88)80029-7)
Y. Shizuri, M. Ohkubo, S. Yamamura, Tetrahedron Lett. 30 (1989) 3797 (https://doi.org/10.1016/S0040-4039(01)80658-4)
M. Franck-Neumann, M. Miesch, L. Gross, Tetrahedron Lett. 32 (1991) 2135 (https://doi.org/10.1016/S0040-4039(00)71256-1)
J. K. Dickson, Jr., B. Fraser-Ried, J. Chem. Soc. Chem. Commun. (1990) 1440 (https://doi.org/10.1039/C39900001440)
V. K. Singh, T. K. Chakraborty, Chem. Asian J. 16 (2021) 753 (https://doi.org/10.1002/asia.202100144)
a) L. A. Paquette, R. A. J. Galemmo, J. P. Springer, J. Am. Chem. Soc. 105 (1983) 6975 (https://doi.org/10.1021/ja00361a046); b) L. A. Paquette, R. A. Galemmo Jr, J. C. Caille, R. S. Valpey, J. Org. Chem. 51 (1986) 686 (https://doi.org/10.1021/jo00355a019)
T. Tsunoda, Y. Kabasawa, S. Itô, M. Kodama, Tetrahedron Lett. 25 (1984) 773 (https://doi.org/10.1016/S0040-4039(01)80023-X)
a) P. D. Magnus, M. S. Nobbs, Synth. Commun. 10 (1980) 273 (https://doi.org/10.1080/00397918008062750) ; b) D. Pauley, F. Anderson, T. Hudlicky, Org. Synth. 67 (1989) 121 (https://doi.org/10.15227/orgsyn.067.0121) ; c) J. Yang, Y. O. Long, L. A. Paquette, J. Am. Chem. Soc. 125 (2003) 1567 (https://doi.org/10.1021/ja021177r)
J. Richers, A. Pöthig, E. Herdtweck, C. Sippel, F. Hausch, K. Tiefenbacher, Chem. Eur. J. 23 (2017) 3178 (https://doi.org/10.1002/chem.201605362).