Ammonia removal by natural and modified clinoptilolite Scientific paper
Main Article Content
Abstract
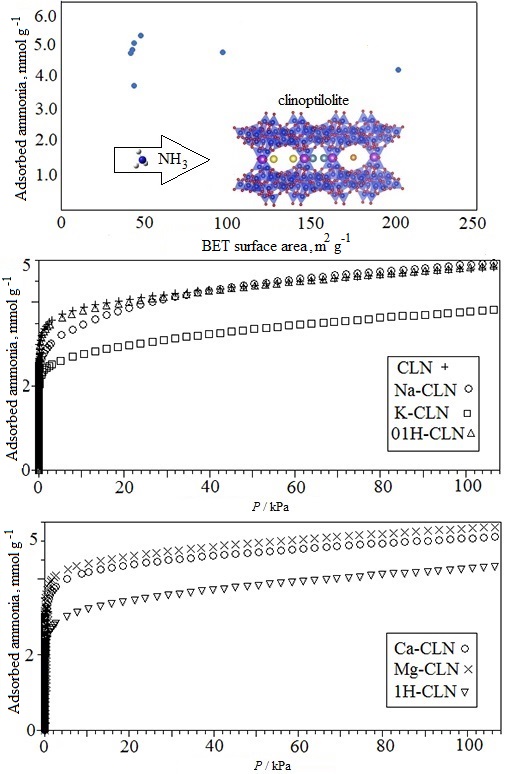
In this study, cation exchange and acid activation processes were applied to determine the effects of different cationic compositions of clinoptilolite on ammonia adsorption properties. Thermogravimetric (TG), differential thermal analysis (DTA), X-ray diffraction (XRD), X-ray fluorescence (XRF) and nitrogen adsorption techniques were used for the characterization of the clinoptilolite samples. As a result of ion exchange and acid activation, the amount, type and location of exchangeable cations in the structure significantly affected the thermal properties, as well as NH3 removal efficiency. Ammonia adsorption isotherms were obtained at 298 K up to 100 kPa volumetrically. In addition, NH3 adsorption capacities of the clinoptilolite samples within this study (3.823 to 5.372 mmol g-1) were compared with those of the other materials (1.77 to 12.2 mmol g-1) in terms of their textural and structural differences.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Anadolu Üniversitesi
Grant numbers 1602F072
References
P. Carson, C. Mumford, Hazardous Chemicals Handbook. Butterworth-Heinemann, Oxford, 2002, pp. 276–279 (https://www.elsevier.com/books/hazardous-chemicals handbook/carson/978-0-7506-4888-2)
N. I. Sax, Dangerous Properties of Industrial Materials, Van Nostrand Reinhold, New York, 1984, p. 1251 (https://aiche.onlinelibrary.wiley.com/doi/abs/10.1002/aic.690260134)
Toxicological Review of Ammonia Noncancer Inhalation: Executive Summary, 2020 (https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0422_summary.pdf, Accessed 26 August 2021)
F. B. Eddy, in Water/Air Transitions in Biology, A. K. Mittal, F. B. Eddy, J. S. Datta Munshi, Eds., Science Publishers Inc, Enfield, NH, 1999, p. 281 (https://www.amazon.com/Water-Air-Transition-Biology-Mittal/dp/1578080592)
H. H. Kristensen, C. Wathes, Worlds Poult. Sci. J. 56 (2000) 235 (https://doi.org/10.1079/WPS20000018)
E. F. Wheeler, K. D. Casey, R. S. Gates, H. Xin, J. L. Zajaczkowski, P. A. Topper, Y. Liang, A. J. Pescatore, Trans. ASABE 49 (2006) 1495 (https://doi.org/10.13031/2013.22042)
D. M. Miles, S. L. Branton, B. D. Lott, Poultry Sci. 83 (2004) 1650 (https://doi.org/10.1093/ps/83.10.1650)
K. J. Donham, D. Cumro, S. Reynolds, J. Agromed. 8 (2002) 57 (https://doi.org/10.1300/J096v08n02_09)
D. W. Breck, Zeolite Molecular Sieves, Wiley, New York 1984 (https://books.google.com.tr/books/about/Zeolite_Molecular_Sieves.html?id=aY0vAQAAIAAJ&redir_esc=y)
G. Gottardi, E. Galli, Natural zeolites, Springer, Berlin, 1985 (https://link.springer.com/book/10.1007/978-3-642-46518-5)
M. W. Ackley, R. F. Giese, R. T. Yang, Zeolites 12 (1992) 780 (https://doi.org/10.1016/0144-2449(92)90050-Y)
K. Koyama, Y. Takeuchi, Z. Krist. 145 (1977) 216 (https://doi.org/10.1524/zkri.1977.145.3-4.216)
T. Armbruster, M. E. Gunter, in Reviews in Mineralogy and Geochemistry, Natural Zeolites: Occurrences, Properties, Appli¬ca¬tions, D. L. Bish, D. W. Ming, Eds. Mineralogical Society of America, Washington DC, 2001, p. 1 (https://pubs.geoscienceworld.org/msa/rimg/article-abstract/45/1/1/140719/Crystal-Structures-of-Natural-Zeolites?redirectedFrom=fulltext)
E. Kouvelos, K. Kesore, T. Steriotis, H. Grigoropoulou, D. Bouloubasi, N. Theophilou, S. Tzintzos, N. Kanelopoulos, Micropor. Mesopor. Mater. 99 (2007) 106 (https://doi.org/10.1016/j.micromeso.2006.07.036)
J. Helminen, J. Helenius, E. Paatero, I. Turunen, J. Chem. Eng. Data 46 (2001) 391 (https://doi.org/10.1021/je000273+)
L. Benco, D. Tunega, Phys. Chem. Miner. 36 (2009) 281 (https://doi.org/10.1007/s00269-008-0276-9)
D. Saha, S. Deng, J. Colloid Interface Sci. 345 (2010) 402 (https://doi.org/10.1016/j.jcis.2010.01.076)
D. Saha, S. Deng, J. Chem. Eng. Data 55 (2010) 5587 (https://doi.org/10.1021/je100405k)
D. Saha, S. Deng, J. Colloid Interface Sci. 348 (2010) 615 (https://doi.org/10.1016/j.jcis.2010.04.078)
D.T. Hayhurst, Chem. Eng. Commun. 4 (1980) 729 (https://doi.org/10.1080/¬0098644¬8008935944)
D. Kallo, J. Papp, J. Valyon, Zeolites 2 (1982) 13 (https://doi.org/10.1016/S0144-2449(82)80034-1)
C. C. Huang, H. S. Li, C. H. Chen, J. Hazar. Mater. 159 (2008) 523 (https://doi.org/10.1016/j.jhazmat.2008.02.051)
H. Asilian, S. B. Mortazav, H. Kazemian, S. Phaghihzadeh, Sj. Shahtaheri, M. Salem, Iran J. Public Health 33 (2004) 45 (https://ijph.tums.ac.ir/index.php/ijph/article/view/1929)
D. Caputo, B. De Gennaro, B. Liguori, M. Pansini, C. Colella, Stud. Surf. Sci. Catal. 140 (2001) 121 (https://doi.org/10.1016/S0167-2991(01)80142-7)
K. Ciahotny, L. Melenova, H. Jirglova, O. Pachtova, M. Kocirık, M. Eic, Adsorption 12 (2006) 219 (https:// doi.org/ 10.1007/s10450-006-0148-x)
F. Esenli, A. Sirkecioğlu, Clay Miner. 40 (2005) 557 (https://doi.org/10.1180/0009855054040192)
G. E. Christidis, D. Moraeti, E. Keheyan, L. Akhalbedashvili, N. Kekelidze, R. Gevorkyan, H. Yeritsyan, H. Sargsyan, Appl. Clay Sci. 24 (2003) 79 (https://doi.org/10.1016/S0169-1317(03)00150-9)
Radosavljević-Mihajlović, V. Dondur, A. Daković, J. Lemic, M. Tomašević-Čano-vić, J. Serb. Chem. Soc. 69 (2004) 273 (https://doi.org/10.2298/JSC0404273R)
S. Lowell, J.E. Shields, M.A. Thomas, M. Thommes, Characterization of porous solids and powders: surface area, pore size and density, Springer, Amsterdam, 2006 (https://link.springer.com/book/10.1007/978-1-4020-2303-3)
D. L. Bish, in Occurrence, properties and utilization of natural zeolites, D. Kallo, H. S. Sherry, Eds., Akademiai Kiado, Budapest, 1988, p. 565 (https://books.google.com.tr/books/about/Occurrence_Properties_and_Utilization_of.html?id=e1LwAAAAMAAJ&redir_esc=y)
S. Moribe, Z. Chen, S. Alayoglu, Z. H. Syed, T. Islamoglu, O. K. Farha, ACS Mater. Lett. 1 (2019) 476 (https://doi.org/10.1021/acsmaterialslett.9b00307)
M. J. Katz, A.J. Howarth, P.Z. Moghadam, J.B. DeCoste, R.Q. Snurr, J. T. Hupp, O. K. Farha, Dalton Trans. 45 (2016) 4150 (https://doi.org/10.1039/C5DT03436A)
K. Ciahotny, L. Melenova, H. Jirglova, M. Boldis, M. Kocirik, in Studies in Surface Science and Catalysis, Vol. 142, R. Aiello, G. Giordano, F. Testa, Eds., Elsevier Science, Amsterdam, 2002, p. 1713 (https://doi.org/10.1016/S0167-2991(02)80344-5).