Monte Carlo optimization based QSAR modeling of the cytotoxicity of acrylic acid-based dental monomers Scientific paper
Main Article Content
Abstract
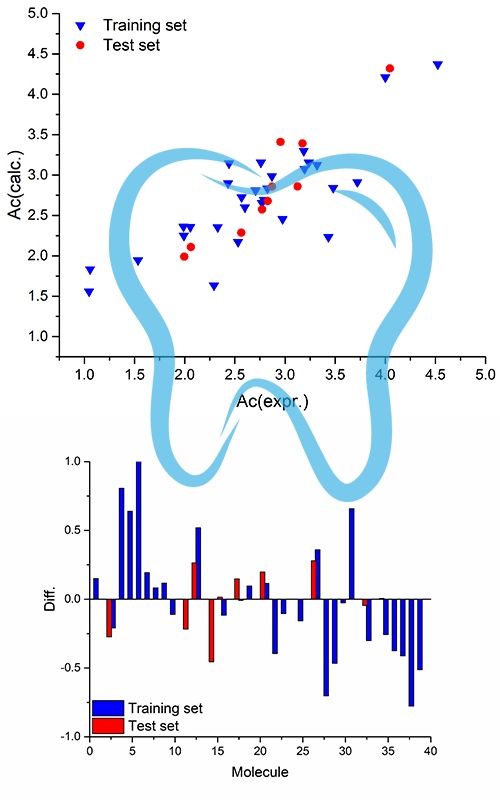
Acrylic acid derivatives are extensively utilized as initial monomers in dental materials. Nevertheless, these substances exhibit cytotoxicity towards different cell types, a phenomenon that must be reduced in future materials. The primary objective of this research is to establish a QSAR model for the prediction of cytotoxic effects and to identify molecular fragments and descriptors with mechanistic interpretations that play a role in cytotoxic effects. The Monte Carlo optimization technique employed QSAR models that are not reliant on conformation. These models utilized both molecular graph-based and SMILES-based descriptors. By employing a variety of statistical methodologies, an assessment of the predictive capabilities and resilience of the established QSAR models was achieved. The demonstrated numerical values used for their validation underscore the strong suitability of these QSAR models. The Monte Carlo optimization technique effectively identified molecular fragments represented in QSAR modeling through the use of SMILES notation, elucidating their impact on cytotoxicity, both positively and negatively. Given that the majority of molecular databases adhere to this molecular structure conformation, the featured QSAR models can serve as a rapid and precise screening tool for novel dental monomers.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200113
References
I. M. Barszczewska-Rybarek, Materials (Basel) 12 (2019) 4057 (https://doi.org/10.3390/ma12244057)
N. Moszner, T. Hirt, J. Polym. Sci. Pol. Chem. 50 (2012) 4369 (https://doi.org/10.1002/pola.26260)
D. Dressano, M. V. Salvador, M. T. Oliveira, G. M. Marchi, B. M. Fronza, M. Hadis, W. M. Palin, A. F. Lima, J. Mech, Behav. Biomed. Mater. 110 (2020) 103875 (https://doi.org/10.1016/j.jmbbm.2020.103875)
R. Gautam, R. D. Singh, V. P. Sharma, R. Siddhartha, P. Chand, R. Kumar, J. Biomed. Mater. Res. B Appl. Biomater. 100 (2012) 1444 (https://doi.org/10.1002/jbm.b.32673)
E. C. Kim, H. Park, S. I. Lee, S. Y. Kim, Basic Clin. Pharm. 117 (2015) 340 (https://doi.org/10.1111/bcpt.12404)
M. Goldberg, Clin. Oral Investig. 12 (2008) 1 (https://doi.org/10.1007/s00784-007-0162-8)
S. K. Jun, J. R. Cha, J. C. Knowles, H. W. Kim, J. H. Lee, H. H. Lee, Dent. Mater. 36 (2020) 157 (https://doi.org/10.1016/j.dental.2019.11.016)
J. G. Leprince, W. M. Palin, M. A. Hadis, J. Devaux, G. Leloup, Dent. Mater. 29 (2013) 139 (https://doi.org/10.1016/j.dental.2012.11.005)
T. Hampe, A. Wiessner, H. Frauendorf, M. Alhussein, P. Karlovsky, R. Bürgers, S. Krohn, Polymers (Basel) 14 (2022) 1790 (https://doi.org/10.3390/polym14091790)
L. S. Mokeem, I. M. Garcia, M. A. Melo, Biomedicines 11 (2023) 1256 (https://doi.org/10.3390/biomedicines11051256)
F. Amin, M. A. Fareed, M. S. Zafar, Z. Khurshid, P. J. Palma, N. Kumar, Coatings 12 (2022) 1094 (https://doi.org/10.3390/coatings12081094)
S. Bandarra, P. Mascarenhas, A. R. Luis, M. Catrau, E. Bekman, A. C. Ribeiro, S. Félix, J. Caldeira, I. Barahona, Clin. Oral Investig. 24 (2020) 2691 (https://doi.org/10.1007/s00784-019-03131-4)
A. Bakopoulou, T. Papadopoulos, P. Garefis, Int. J. Mol. Sci. 10 (2009) 3861 (https://doi.org/10.3390/ijms10093861)
M. Cadenaro, T. Maravic, A. Comba, A. Mazzoni, L. Fanfoni, T. Hilton, J. Ferracane, L. Breschi, Dent. Mater. 35 (2019) e1 (https://doi.org/10.1016/j.dental.2018.11.012)
G. Cervino, M. Cicciù, A. S. Herford, A. Germanà, L. Fiorillo, Materials (Basel) 13 (2020) 3350 (https://doi.org/10.3390/ma13153350)
I. P. Caldas, G. G. Alves, I. B. Barbosa, P. Scelza, F. de Noronha, M. Z. Scelza, Dent. Mater. 35 (2019) 195-205 (https://doi.org/10.1016/j.dental.2018.11.028)
E. N. Muratov, J. Bajorath, R. P. Sheridan, I. V. Tetko, D. Filimonov, V. Poroikov, T. I. Oprea, I. I. Baskin, A. Varnek, A. Roitberg, O. Isayev, S. Curtarolo, D. Fourches, Y. Cohen, A. Aspuru-Guzik, D. A. Winkler, D. Agrafiotis, A. Cherkasov, A.Tropsha, Chem. Soc. Rev. 49 (2020) 3525 (https://doi.org/10.1039/d0cs90041a)
P. Liu, W. Long, Int. J. Mol. Sci. 10 (2009) 1978 (https://doi.org/10.3390/ijms10051978)
N. Tripathi, M. K. Goshisht, S. K. Sahu, C. Arora, Mol. Divers. 25 (2021) 1643 (https://doi.org/10.1007/s11030-021-10237-z)
А. P. Toropova, A. A. Toropov, Mini Rev. Med. Chem. 18 (2018) 382 (https://doi.org/10.2174/1389557517666170927154931)
А. M. Veselinović, J. B. Veselinović, J. V. Živković, G. M. Nikolić, Curr. Top. Med. Chem. 15 (2015) 1768 (https://doi.org/10.2174/1568026615666150506151533)
A. K. Halder, A. H. S. Delgado, M. N. D. S. Cordeiro, Dent. Mater. 38 (2022) 333 (https://doi.org/10.1016/j.dental.2021.12.014)
P. K. Ojha, K. Roy, Chemometr. Intell. Lab. 109 (2011) 146 (https://doi.org/10.1016/j.chemolab.2011.08.007)
V. Stoičkov, D. Stojanović, I. Tasić, S. Šarić, D. Radenković, P. Babović, D. Sokolović, A. M. Veselinović, Struct. Chem. 29 (2018) 441 (https://doi.org/10.1007/s11224-017-1041-9)
А. A. Toropov, A. P. Toropova, Mutat. Res.-Gen. Tox. En. 819 (2017) 31 (https://doi.org/10.1016/j.mrgentox.2017.05.008)
A. M. Veselinović, A. Toropov, A. Toropova, D. Stanković-Đorđević, J. B. Veselinović, New J. Chem. 42 (2018) 10976 (https://doi.org/10.1039/C8NJ01034J)
P. K. Ojha, K. Roy, Chemometr. Intell. Lab. Syst. 109 (2011) 146 (https://doi.org/10.1016/j.chemolab.2011.08.007)
P. K. Ojha, I. Mitra, R. N. Das, K. Roy, Chemometr. Intell. Lab. Syst. 107 (2011) 194 (https://doi.org/10.1016/j.chemolab.2011.03.011)
P. P. Roy, J. T. Leonard, K. Roy, Chemometr. Intell. Lab. Syst. 90 (2008) 31 (https://doi.org/10.1016/j.chemolab.2007.07.004)
K. Roy, R. N. Das, P. Ambure, R. B. Aher. Chemometr. Intell. Lab. 152 (2016) 18 (https://doi.org/10.1016/j.chemolab.2016.01.008)
L. I. Lin, Biometrics 45 (1989) 255 (https://doi.org/10.2307/2532051)
O. Nicolotti, D. Gadaleta, G. F. Mangiatordi, M. Catto, A. Carotti, IJQSPR 1 (2016) 45 (https://doi.org/10.4018/IJQSPR.2016010102)
P. Gramatica, QSAR Comb. Sci. 26 (2007) 694 (https://doi.org/10.1002/qsar.200610151)
P. Gramatica, A. Sangion, J. Chem. Inf. Model. 56 (2016) 1127 (https://doi.org/10.1021/acs.jcim.6b00088)
M. Zivkovic, M. Zlatanovic, N. Zlatanovic, M. Golubović, A. M. Veselinović, Mini Rev. Med. Chem. 20 (2020) 1389 (https://doi.org/10.2174/1389557520666200212111428)
S. A. Amin, N. Adhikari, S. Gayen, T. Jha, J. Biomol. Struct. Dyn. 37 (2019) 4528 (https://doi.org/10.1080/07391102.2018.1552895)
S. Ahmadi, S. Lotfi, S. Afshari, P. Kumar, E. Ghasemi, SAR QSAR Environ. Res. 32 (2021) 1013 (https://doi.org/10.1080/1062936X.2021.2003429).