Advanced dye removal by multifunctional layered double hydroxide based materials: Adsorption and kinetic studies Scientific paper
Main Article Content
Abstract
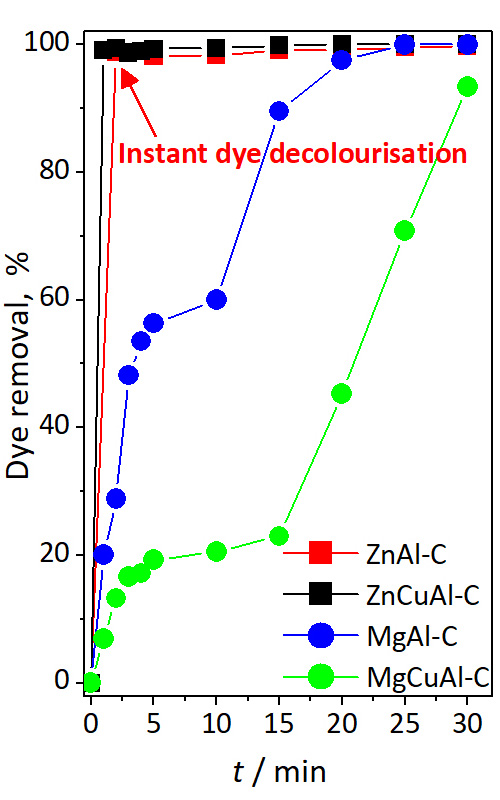
Due to favourable properties layered double hydroxides (LDHs) have been widely investigated for organic dye removal processes. In order to study the adsorption of methyl orange, bimetal (ZnAl and MgAl) and trimetal (ZnCuAl and MgCuAl) adsorbents were synthesized and thermally treated. The influence of adsorbent metal nature and content on structural (X-ray diffraction, Raman analysis), textural (low temperature nitrogen adsorption) and adsorption properties was investigated. Adsorption behaviour, mechanisms, and stability of synthesized LDHs and their calcined mixed oxides were studied with the aim to elucidate the adsorbent-dye interactions, enabling optimization of experimental design. All LDH adsorbents and LDH derived mixed oxide adsorbents had high removal efficiency rate, especially Zn-containing mixed oxides where complete decolourization (100 % of dye removal) was achieved almost instantly due to super-fast adsorbent-adsorbate interaction. Two possible adsorption mechanisms initiated by interfacial phenomena were in correlation with the structural and textural properties, as well as with the “memory effect” reconstruction phenomenon. These results present a solid base for further investigation and design of LDH-based adsorbents for the Methyl orange removal, considering their favourable structural and textural properties and excellent adsorption capacities.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200134 -
Provincial Secretariat for Higher Education and Scientific Research, Autonomous Province of Vojvodina
Grant numbers 142-451-2341/2021-01/02
References
H.-Y. Xu, B. L. Ping Li, J. Serb. Chem. Soc. 83 (2018) 1261 (https://doi.org/10.2298/JSC180501060X)
N. Li, Z. Chang, H. Dang, Y. Zhan, J. Lou, S. Wang, S. Attique, W. Li, H. Zhou, C. Sun, Colloids Surfaces, A. 591 (2020) 124507 (https://doi.org/10.1016/j.colsurfa.2020.124507)
T. Guan, L. Fang, Y. Lu, F. Wu, F. Ling, J. Gao, B. Hu, F. Meng, X. Jin, Colloids Surfaces, A 529 (2017) 907 (http://dx.doi.org/10.1016/j.colsurfa.2017.06.049)
M. Abniki, A. Moghimi, F. Azizinejad, J. Serb. Chem. Soc. 85 (2020) 1223 (https://doi.org/10.2298/JSC191011004A)
M. Hadnadjev-Kostic, T. Vulic, R. Marinkovic-Neducin, Adv. Powder Technol. 25 (2014) 1624 (https://doi.org/10.1016/j.apt.2014.05.015)
M. Hadnadjev-Kostic, T. Vulic, R. Marinkovic-Neducin, D. Loncarevic, J. Dostanic, S. Markov, D. Jovanovic, J. Clean. Prod. 164 (2017) 1 (https://doi.org/10.1016/j.jclepro.2017.06.091)
D. Bharali, R. Dekam, Colloids Surfaces, A 525 (2017) (https://doi.org/10.1016/j.colsurfa.2017.04.060)
K. Hassani, B. Beakou, D. Kalnina, E. Oukani, A. Anouar, Appl. Clay Sci. 140 (2017) 124 (https://doi.org/10.1016/j.clay.2017.02.010)
L. Zhang, J. Liu, H. Xia, D. Liu, Y. Qin, H. Wu, H. Li, N. Du, W. Hou, Chem. Eng. J. 250 (2014) 1 (https://doi.org/10.1016/j.cej.2014.03.098)
L. Wu, B. Peng, Q. Li, Q. Wang, X. Yan, K. Li, Q. Lin, New J. Chem. 44 (2020) 5293 (https://doi.org/10.1039/D0NJ00278J)
X. Cheng, X. Huang, X. Wang, D. Sun, J. Hazard. Mater. 177 (2010) 516 (https://doi.org/10.1016/j.jhazmat.2009.12.063)
N. Pathak, S. K. Gupta, L. Prajapat, S. K. Sharma, P. S. Ghosh, B. Kanrar, P. K. Pujariab, R. M.Kadam, Phys. Chem. Chem. Phys. 19 (2017) 11975 (https://doi.org/10.1039/C7CP01776F)
M. Jablonska, L. Chmielarz, A. Wegrzyn, K. Guzik, Z. Piwowarska, S. Witkowski, R. I. Walton, P. W. Dunne, F. Kovanda, J. Therm. Anal. Calorim. 114 (2013) 731 (https://doi.org/10.1007/s10973-012-2935-9)
A. A. A. Ahmed, Z. A. Talib, M. Z. Bin Hussein, Appl. Clay Sci. 56 (2012) 68 (https://doi.org/10.1016/j.clay.2011.11.024)
K. Morimoto, K. Tamura, N. Iyi, J. Ye, H. Yamada, J. Phys. Chem. Solids 72 (2011) 1037 (https://doi.org/10.1016/j.jpcs.2011.05.018)
L. Wu, B. Peng, Q. Li, Q. Wang, X. Yan, K. Li, Q. Lin, New J. Chem. 44 (2020) 5293 (https://doi.org/10.1039/D0NJ00278)
I. M. Ahmed, M. S. Gasser, Appl. Surf. Sci. 259 (2012) 650 (https://doi.org/10.1016/j.apsusc.2012.07.092)
R. A. B. Lima-Correa, C. S. Castro, A. S. Damasceno, J. M. Assaf, Renew. Energy 146 (2020) 1984 (https://doi.org/10.1016/j.renene.2019.08.047)
E.M. Seftel, R.G. Ciocarlan, B. Michielsen, V. Meynen, S. Mullens, P. Cool, Appl. Clay Sci. 165 (2018) 234 (https://doi.org/10.1016/j.clay.2018.08.018)
S. Kim, J. Fahel, P. Durand, E. André, C. Carteret, Eur. J. Inorg. Chem. (2017) 669 (https://doi.org/10.1002/ejic.201601213)
H. Zaghouane-Boudaiaf, M. Boutahala, L. Arab, Chem. Eng. J. 187 (2012) 142 (https://doi.org/10.1016/j.cej.2012.01.112)
C. Lei, X. Zhu, C. Zhu, B. Jiang, Y. Le, J. Yu, J. Hazard. Mater. 321 (2017) 801 (https://doi.org/10.1016/j.jhazmat.2016.09.070)
Z. Gao, K. Sasaki, X. Qiu, Langmuir 34 (2018) 5386 (https://doi.org/10.1021/acs.langmuir.8b00059)
K. L. Tan, B. H. Hameed, J. Taiwan Inst. Chem. Eng. 74 (2017) 25 (https://doi.org/10.1016/j.jtice.2017.01.024)
C. Peng, J. Dai, J. Yu, J. Yin, AIP Advances 5 (2015) 057138 (https://doi.org/10.1063/1.4921455)
Z.-M. Ni, S.-J. Xia, L.-G. Wang, F.-F. Xing, G.-X. Pan, J. Colloid Interface Sci. 316 (2007) 284 (https://doi.org/10.1016/j.jcis.2007.07.045)
Y. Guo, Z. Zhu,Y. Qiu, J. Zhao, Chem. Eng. J. 219 (2013) 69 (https://doi.org/10.1016/j.cej.2012.12.084).