3D-QSAR and molecular docking studies of aminothiazole derivatives as Lim kinase 1 inhibitors Scientific paper
Main Article Content
Abstract
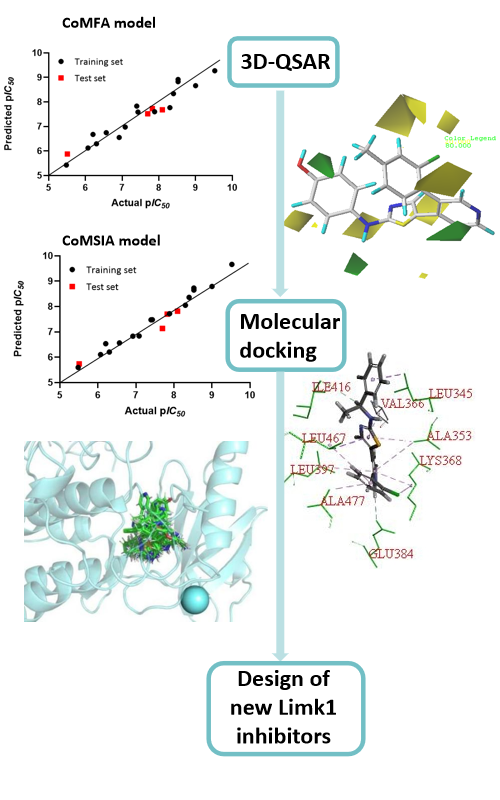
Lim kinase (Limk), as an important cytoskeletal regulator, plays an important role in cancer manifestations and neuronal diseases. Limk1 is a member of the Limk family, which is mainly involved in the invasion and metastasis of tumor cells and is abnormally expressed in a variety of cell carcinoma tissues. In this paper, a series of Limk1 inhibitors with aminothiazole skeleton were used to design potent and efficient Limk1 inhibitors by computational approaches. Firstly, the 3D-QSAR model was constructed, and both CoMFA and CoMSIA models have good correlation and prediction ability. The binding requirements between ligand and receptor protein were then further explored through molecular docking, including the critical forces between Limk1 inhibitors and active site residues. Finally, based on the 3D-QSAR model and molecular docking results analysis, three new compounds with theoretically better activity were designed and their ADME properties were predicted.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Jiangsu Provincial Department of Education
Grant numbers JSSCBS20211302 -
Jiangsu Ocean University
-
Key Laboratory of Coast Ocean Resources Development and Environment Security
Grant numbers JSIMR202015
References
K. Yoshioka, V. Foletta, O. Bernard, K. Itoh, Proc. Natl. Acad. Sci. 100 (2003) 7247 (https://doi.org/10.1073/pnas.1232344100)
C. Prunier, R. Prudent, R. Kapur, K. Sadoul, L. Lafanechère, Oncotarget 8 (2017) 41749 (https://doi.org/10.18632/oncotarget.16978)
F. Yi, J. Guo, D. Dabbagh, M. Spear, S. He, K. Kehn-Hall, J. Fontenot, Y. Yin, M. Bibian, C. M. Park, J. Virol. 91 (2017) e02418-16 (https://doi.org/10.1128/JVI.02418-16)
F. Manetti, Drug Discov. Today 17 (2012) 81 (https://doi.org/10.1016/j.drudis.2011.08.004)
A. Jayo, M. Parsons, J. C. Adams, BMC Biol. 10 (2012) 1 (https://doi.org/10.1186/1741-7007-10-72)
D. H. Vlecken, C. P. Bagowski, Zebrafish 6 (2009) 433 (https://doi.org/10.1089/zeb.2009.0602)
O. Bernard, Int. J. Biochem. Cell Biol. 39 (2007) 1071 (https://doi.org/10.1016/j.biocel.2006.11.011)
P. Chen, M. Zeng, Y. Zhao, X. Fang, Oncol. Rep. 32 (2014) 2070 (https://doi.org/10.3892/or.2014.3461)
J. Zhou, R. Liu, C. Luo, X. Zhou, K. Xia, X. Chen, M. Zhou, Q. Zou, P. Cao, K. Cao, Cancer Biol. Ther. 15 (2014) 1340 (https://doi.org/10.4161/cbt.29821)
P. Ross-Macdonald, H. de Silva, Q. Guo, H. Xiao, C.-Y. Hung, B. Penhallow, J. Markwalder, L. He, R. M. Attar, T. Lin, Mol. Cancer Ther. 7 (2008) 3490 (https://doi.org/10.1158/1535-7163.MCT-08-0826)
B. E. Sleebs, G. Nikolakopoulos, I. P. Street, H. Falk, J. B. Baell, Bioorg. Med. Chem. Lett. 21 (2011) 5992 (https://doi.org/10.1016/j.bmcl.2011.07.050)
Y. Yin, K. Zheng, N. Eid, S. Howard, J.-H. Jeong, F. Yi, J. Guo, C. M. Park, M. Bibian, W. Wu, J. Med. Chem. 58 (2015) 1846 (https://doi.org/10.1021/jm501680m)
M. D. Charles, J. L. Brookfield, T. C. Ekwuru, M. Stockley, J. Dunn, M. Riddick, T. Hammonds, E. Trivier, G. Greenland, A. C. Wong, J. Med. Chem. 58 (2015) 8309 (https://doi.org/10.1021/acs.jmedchem.5b01242)
K. Mardilovich, M. Baugh, D. Crighton, D. Kowalczyk, M. Gabrielsen, J. Munro, D. R. Croft, F. Lourenco, D. James, G. Kalna, Oncotarget 6 (2015) 38469 (https://doi.org/10.18632/oncotarget.6288)
M. Zhang, J. Tian, R. Wang, M. Song, R. Zhao, H. Chen, K. Liu, J.-H. Shim, F. Zhu, Z. Dong, Front. Cell Dev. Biol. (2020) 1361 (https://doi.org/10.3389/fcell.2020.556532)
X. Xu, B. Xu, X. Wang, & J. Li, J. Mol. Struct. 1201 (2020) 127128 (https://doi.org/10.1016/j.molstruc.2019.127128)
M. Sari-Hassoun, M.-J. Clement, I. Hamdi, G. Bollot, C. Bauvais, V. Joshi, F. Toma, A. Burgo, M. Cailleret, M. C. Rosales-Hernández, Biochem. Pharmacol. 102 (2016) 45 (https://doi.org/10.1016/j.bcp.2015.12.013)
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7 (2017) 1 (https://doi.org/10.1038/srep42717)
W. J. Egan, K. M. Merz, J. J. Baldwin, J. Med. Chem. 43 (2000) 3867 (https://doi.org/10.1021/jm000292e).