Highly efficient functional materials for modern electrochemical devices Scientific paper
Main Article Content
Abstract
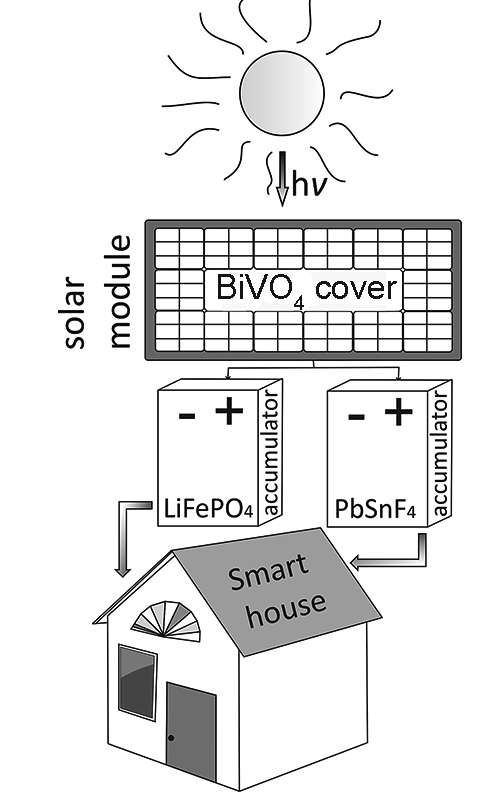
In order to find new functional materials and materials with improved performance for next-generation electrochemical devices, several new materials for various purposes have been synthesized. In particular, BiVO4 films were obtained by electrochemical synthesis using interferometric control of film thickness during their deposition. Previously, it was found that the use of thin BiVO4 films with a thickness of 150 to 400 nm is most effective, where an increase in the quantum yield of photocurrent up to 0.25 at λ of 400 to 450 nm was observed. LiFePO4 was synthesized in DES medium (low-temperature eutectic solvents): choline chloride–triethylene glycol (ChCl–TEG) and choline chloride–ethylene glycol (ChCl–EG) using NH4FePO4 and CH3COOLi as precursors. It was found that the mode of synthesis of LiFePO4/C at 973 К for 1 h does not lead to oxidation of LiFePO4, as evidenced by the values of the ratio Fe2+/Fe3+ for LiFePO4 and LiFePO4/C, which are 2.4 and 2.7, respectively. It was found that the substitution of a part of lead cations (up to 20 mol. %), in the composition of the fluoride-conducting phase Pb0.86Sn1.14F4, contributes to the increase of its conductivity in the whole temperature range, the higher the concentration of the substituent, to a greater extent. Charge transfer is provided by highly mobile interstitial fluorine anions, the concentration of which increases with the rise of temperature and substituent content.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
N. Nitta, F. Wu, Mater. Today 18 (2015) 252 (https://doi.org/10.1016/j.mattod.2014.10.040)
T. L. Kulova, Russ. J. Electrochem. 49 (2013) 25 (https://doi.org/10.1134/S1023193513010102)
X. Kang1, Chem. Rev. 104 (2004) 4303 (https://doi.org/10.1021/cr030203g)
M. S. Whittingham, Chem. Rev. 104 (2004) 4271 (https://doi.org/10.1021/cr020731c)
A. B. Yaroslavtsev, T. L. Kulova, Uspekhi khimii 84 (2015) 826 (https://doi.org/10.1070/RCR4497)
X. Li, B. Yue, App. Catal., A 390 (2010) 2195 (https://doi.org/10.1016/j.apcata.2010.10.013)
G. Teran-Escobar, J. Pampel, Energy Environ. Sci. 6 (2013) 3088 (https://doi.org/10.1039/C3EE42204F)
Z. Yang, Y. Li, J. Catal. 280 (2011) 247 (https://doi.org/10.1016/j.jcat.2011.03.026)
H. Miyake, H. Kozuka, J. Phys. Chem., B 109 (2005) 17951 (https://doi.org/10.1021/jp058051b)
N. I. Sorokin, B. P. Sobolev, Kristallografiya 52 (2007) 870 (https://naukarus.com/nestehiometricheskie-ftoridy-tverdye-elektrolity-dlya-elektrohimicheskih-ustroystv-obzor)
L. N. Patro, K. Hariharan, Solid State Ionics 239 (2013) 41 (https://doi.org/10.1016/j.ssi.2013.03.009)
V. Trnovtsova, P. P. Fedorov, Elektrokhimiya 45 (2009) 668 (https://naukarus.com/ftoridnye-tverdye-elektrolity)
J. Resasco, H. Zhang, ACS Cent. Sci. 2 (2016) 80 (https://doi.org/10.1021/acscentsci.5b00402)
J. Gan, X. Lu, Nanoscale 16 (2014) 7142 (https://doi.org/10.1039/C4NR01181C)
V. O. Smilyk, S. S. Fomanyuk, G. Ya. Kolbasov, I. A. Rusetskyi, V. S. Vorobets, Res. Chem. Intermed. 45 (2019) 4149 (https://doi.org/10.1007/s11164-019-03897-y)
Y. Li, J. Zhu, Sci. Chin. Chem. 9 (2014) 1489 (https://doi.org/10.1007/s11426-015-5348-3)
Z. Yang, Y. Dai, J. Mater. Chem., A 4 (2016) 8210 (https://doi.org/10.1039/C6TA05048D)
L. X. Yuan, Z. H. Wang, Energy Environ. Sci. 4 (2011) 269 (https://doi.org/10.1039/C0EE00029A)
U. S. Kasavajjula, C. Wang, J. Electrochem. Soc. 155 (2008) A866 (https://doi.org/10.1149/1.2980420)
T. V. Satyavani, A. K. Srinivas, Eng. Sci. Tech. 19 (2016) 178 (https://doi.org/10.1016/j.jestch.2015.06.002)
T. Kodera, B. Dongying, Key Eng. Mater. 485 (2011) 107 (https://doi.org/10.4028/www.scientific.net/KEM.485.107)
Y. Lin, M. X. Gao, J. Power Sources 184 (2008) 444 (https://doi.org/10.1016/j.jpowsour.2008.03.026)
V. O. Smilyk, S. S. Fomanyuk, Ukrayinsʹkyy khimichnyy zhurnal 85 (2019) 83 (https://doi.org/10.33609/0041-6045.85.10.2019.83–90)
Ye.V. Kuzminskii, G.Ya. Kolbasov, Sol. Energ. Mater. Sol. Cells 56 (1999) 93 (https://doi.org/10.1016/S0927-0248(98)00146-9)
T. Saison, N. Chemin, J. Phys. Chem. 119 (2015) 12967 (https://doi.org/10.1021/acs.jpcc.5b01468)
R. Katoh, M. Kasuya, Chem. Phys. Lett. 471 (2009) 280 (https://doi.org/10.1016/j.cplett.2009.02.053)
C. A. Grimes, G. K. Mor, TiO2 Nanotube Arrays. Synthesis, Properties, and Applications, Springer, New York, 2009, p. 358 (https://doi.org/10.1007/978-1-4419-0068-5)
R. Sharma, P.P. Das, Nanotechnology 20 (2009) 075704 (https://doi.org/10.1088/0957-4484/20/7/075704)
C. G. Granqvist, Handbook of Inorganic Electrochromic Materials, Elsevier, Uppsala, 1995 (https://doi.org/10.1016/B978-0-444-89930-9.X5000-4)
N. N. Dinh, D. H. Ninh, J. Nanomat. 2012 (2012) 7 (https://doi.org/10.1155/2012/781236)
M. F. Saenger, T. Höing, Phys. Status Solidi, A 4 (2008) 914 (https://doi.org/10.1002/pssa.200777894)
Yu. V. Pohorenko, R. M. Pshenychnyi, J. Serb. Chem. Soc. 86 (2021) 845 (https://doi.org/10.2298/JSC201124031P)
S. S. Fomanyuk, V. О. Smilyk, Fr.-Ukr. J. Chem.06 (2018) 157 (https://doi.org/10.17721/fujcV6I1P157-166)
Q. Jia, K. Iwashina, Proc. Natl. Acad. Sci. USA 109 (2012) 11564 (https://doi.org/10.1073/pnas.1204623109)
D. K. Lee, K.-S. Choi, Nat. Energy 3 (2018) 53 (https://doi.org/10.1038/s41560-017-0057-0)
M. G. Mali, H. Yoon, Appl. Phys. Lett. 106 (2015) 151603 (https://doi.org/10.1063/1.4918583)
O. Bohnke, C. Bohnke, Solid State Ionics 6 (1982) 267 (https://doi.org/10.1016/0167-2738(82)90048-0)
V. Galaguz, O. Korduban, J. Serb. Chem. Soc. 85 (2020) 1047 (https://doi.org/10.2298/JSC190910011G).