Designing an electrochemical biosensor based on tyrosinase for highly sensitive and rapid detection of bisphenol A and its derivatives Scientific paper
Main Article Content
Abstract
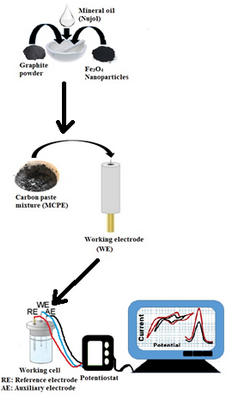
Bisphenol A (BPA) is a monomer commonly used in the production of epoxy resins, plastic bottles and dental filling materials. Due to its chemical structure, BPA and its derivates show activity similar to the endocrine hormones. It can bind to estrogen receptors and cause neurological disturbances, even at low doses. Therefore, it is important to determine BPA and its derivatives quickly and sensitively at low concentrations. In this study, a single amperometric tyrosinase enzyme biosensor was designed for the determination of the amount of BPA, bisphenol F (BPF) and bisphenol S (BPS) monomers. Tyrosinase was immobilized onto a modified carbon paste electrode by cross-linking with glutaraldehyde. The amount of BPA (BPS and BPF) was determined directly on the reduction of quinone compound released as a result of the enzymatic reaction at –0.15V. Km(app) value of the designed biosensor for BPA was found 0.00067 μM, the linear operating range was 0.001–0.005 μM (a) and 0.03–0.1 μM (b) and the lower detection limit was found 1 nM for each monomer. It is clear that designed biosensor enable the fast, efficient and precise determination of BPA and its derivatives released from materials used in dental materials.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Gazi Üniversitesi
Grant numbers 05/2020-11
References
F. Fleisch, P. E. Sheffield, C. Chinn, B. L. Edelstein, P. J. Landrigan, Pediatrics 126 (2010) 760 (https://doi.org/10.1542/peds.2009-2693)
L. A. D. Gugoasa, J. Electrochem. Soc. 167 (2020) 037506 (https://doi.org/10.1149/2.0062003JES)
K. V. Ragavan, N. K. Rastogi, M. S. Thakur, TrAC - Trends Anal. Chem. 52 (2013) 248 (http://doi.org/10.1016/j.trac.2013.09.006)
EFSA, EFSA J. 428 (2006) (https://doi.org/10.2903/j.efsa.2004.86)
X. Lu, X. Wang, L. Wu, L. Wu, L. Fu, Y. Gao, J. Chen, ACS Appl. Mater. Interf. 8 (2016) 16533 (https://doi.org/10.1021/acsami.6b05008)
H. Yin, L. Cui, Q. Chen, W. Shi, S. Ai, L. Zhu, L. Lu, Food Chem. 125 (2011) 1097 (https://doi.org/10.1016/j.foodchem.2010.09.098)
O. C. Bodur, S. Dinç, M. Özmen, F. Arslan, Biotechnol. Appl. Biochem. 68 (2021) 20 (https://doi.org/10.1002/bab.1886)
S. Donmez, F. Arslan, N. Sarı, E. Hasanoğlu Özkan, H. Arslan, Biotechnol. Appl. Biochem. 64 (2017) 745 (https://doi.org/10.1002/bab.1533)
F. Arslan, Sensors 8 (2008) 5492 (https://doi.org/10.3390/s8095492)
P. C. Pwavodi, V. H. Ozyurt, S. Asir, M. Ozsoz, Micromachines 12 (2021) 312 (https://doi.org/10.3390/mi12030312)
P. Deng, Z. Xu, Y. Kuang, Food Chem. 157 (2014) 490 (https://doi.org/10.1016/j.foodchem.2014.02.074)
S. S., Shankar, R. M., Shereema, V., Ramachandran, T. V., Sruthi, V. S., Kumar, R. B. Rakhi, ACS Omega 4 (2019) 7903 (https://doi.org/10.1021/acsomega.9b00230)
S. Wang, Y. Tan, D. Zhao, G. Liu, Biosens. Bioelectron. 23 (2008) 1781 (https://doi.org/10.1016/j.bios.2008.02.014)
X. Xu, Q. Zheng, G. Bai, L. Song, Y. Yao, X. Cao, C. Yao, Electrochim. Acta 242 (2017) 56 (https://doi.org/10.1016/j.electacta.2017.05.007)
E. Aynacı, A. Yaşar, F. Arslan, Sensors Actuators, B 202 (2014) 1028 (https://doi.org/10.1016/j.snb.2014.06.049)
Q. Xin, R. M. Wightman, Brain Res. 776 (1997) 126 (https://doi.org/10.1016/S0006-8993(97)00996-7)
L. Wu, X. Lu, K. Niu, Dhanjai, J. Chen, Biosens. Bioelectron. 165 (2020) 112407 (https://doi.org/10.1016/j.bios.2020.112407)
M. Sýs, M. Obluková, V. Kolivoška, R. Sokolová, L. Korecká, T. Mikysek, J. Electroanal. Chem. 864 (2020) 114066 (https://doi.org/10.1016/j.jelechem.2020.114066)
Erkmen, S. Kurbanoglu, B. Uslu, Sensors Actuators, B 316 (2020) 128121 (https://doi.org/10.1016/j.snb.2020.128121)
M. Najib, M. E. Ghicad, C. Dridia, B. M. Alia, M. A. Christopher, Talanta 184 (2018) 388 (https://doi.org/10.1016/j.talanta.2018.03.031)
F. A. A. Manan, W. W. Hong, J. Abdullah, N. A. Yusof, I. Ahmad, Mater. Sci. Eng., C 99 (2019) 37 (https://doi.org/10.1016/j.msec.2019.01.082)
Wong, A. Santos, O. Fatibello Filho, M. Sotomayor, Electroanalysis 33 (2020) 431 (https://doi.org/10.1002/elan.202060084)
L. A. Mercante, L. E. Iwaki, V. P. Scagion, O. N. Oliveira, L. H. Mattoso, D. S. Correa, Electrochem. 2 (2021) 41 (https://doi.org/10.3390/electrochem2010004)
P. Sun, Y. Wu, Sensors Actuators, B 178 (2013) 113 (https://doi.org/10.1016/j.snb.2012.12.055)
M. Han, Y. Qu, S. Chen, Y. Wang, Z. Zhang, M. Ma, Z. Wang, G. Zhan, C. Li, Microchim. Acta 180 (2013) 989 (https://doi.org/10.1007/s00604-013-1018-3)
J. Kochana, K. Wapiennik, J. Kozak, P. Knihnicki, A. Pollap, M. Woźniakiewicz, P. Kościelniak, Talanta 144 (2015) 163 (https://doi.org/10.1016/j.talanta.2015.05.078)
L.A. Gugoasa, R.I. Stefan-van Staden, J.F. van Staden, M. Coroș, S. Pruneanu, Anal. Lett. 52 (2019) 2583 (https://doi.org/10.1080/00032719.2019.1620262)
R.I. Stefan-van Staden, L.A. Gugoaşă, B. Calenic, J.F. van Staden, J. Legler, Anal. Chem. Res. 1 (2014) 1 (https://doi.org/10.1016/j.ancr.2014.06.001)
B. Arslan, E. Yıldırım, C. O. Bodur, B. B. Tuncer, Ç. M. Ulusoy, C. Tuncer, Turk. J. Orthod. 35 (2022) 27 (https://doi.org/10.5152/TurkJOrthod.2021.21176).