Synthesis and antiproliferative activity of (5R)-cleistenolide and analogues Scientific paper
Main Article Content
Abstract
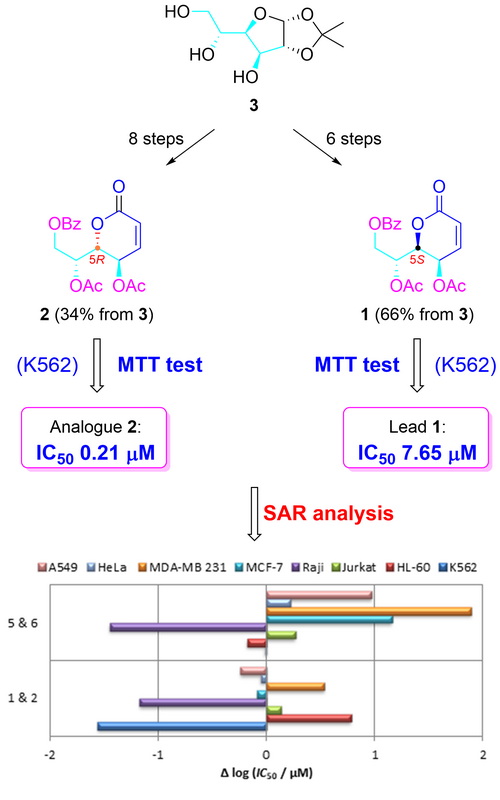
(5R)-Cleistenolide and a few related analogues have been synthesized starting from d-glucose. The key steps of the synthesis included a Z-selective Wittig olefination and an intramolecular Mitsunobu reaction with an inversion of configuration at the C-5 position. In vitro antiproliferative activity of synthesized compounds was tested on a panel of eight human tumour cells and against a single normal cell line (MRC-5). The majority of tested compounds showed strong antiproliferative effects on certain human tumour cells and all of them showed negligible toxicity to normal foetal lung fibroblasts (MRC-5). The most active compound obtained in this work is lactone 5, which in MDA-MB 231 cell culture showed the same activity as doxorubicin (IC50 0.09 µM). Strong antiproliferative activities of analogues 2, 5 and 6 were recorded in the K562 cell line (IC50 0.21, 0.34 and 0.33 µM, respectively), in which they showed very similar activities to doxorubicin (IC50 0.25 µM). A performed SAR study revealed that a change in the stereochemistry at the C-5 position may increase the activity of resulting stereoisomers.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers Contract No. 451-03-68/2020-14/200125 -
Serbian Academy of Sciences and Arts
Grant numbers F-130
References
S. Samwel, S. J. M. Mdachi, M. H. H. Nkunya, B. N. Irungu, M. J. Moshi, B. Moulton, B. Luisi, Nat. Prod. Commun. 2 (2007) 737 (https://doi.org/10.1177/1934578X0700200706)
M. H. H. Nkunya, Pure Appl. Chem. 77 (2005) 1943 (https://doi.org/10.1351/pac200577111943)
F. Pereira, A. M. Madureira, S. Sancha, S. Mulhovo, X. Luo, A. Duarte, M. J. U. Ferreira, J. Ethnopharm. 178 (2016) 180 (https://doi.org/10.1016/j.jep.2015.12.009)
R. Verzár, G. Petri, J. Ethnopharmacol. 19 (1987) 67 (https://doi.org/10.1016/0378-8741(87)90137-1)
G. Benedeković, M. Popsavin, N. S. Radulović, Z. Stojanović-Radić, S. Farkas, J. Francuz, V. Popsavin, Bioorg. Chem. 106 (2021) 104491 (https://doi.org/10.1016/j.bioorg.2020.104491)
G. Benedeković, I. Kovačević, M. Popsavin, J. Francuz, V. Kojić, G. Bogdanović, V. Popsavin, Bioorg. Med. Chem. Lett. 26 (2016) 3318 (https://doi.org/10.1016/j.bmcl.2016.05.044)
G. Benedeković, M. Popsavin, I. Kovačević, V. Kojić, M. Rodić, V. Popsavin, Eur. J. Med. Chem. 202 (2020) 112597 (https://doi.org/10.1016/j.ejmech.2020.112597)
G. Benedeković, M. Popsavin, I. Kovačević, V. Kojić, J. Kesić, S. Farkas, V. Popsavin, Tetrahedron 96 (2021) 132385 (https://doi.org/10.1016/j.tet.2021.132385)
S. S. Nyandoro, G. Maeda, J. J. E. Munissi, A. Gruhonjic, P. A. Fitzpatrick, S. Lindblad, S. Duffy, J. Pelletier, F. Pan, R. Puttreddy, V. M. Avery, M. Erdélyi, Molecules 24 (2019) 2746 (https://doi.org/10.3390/molecules24152746)
P. S. Mahajan, R. G. Gonnade, S. B. Mhaske, Eur. J. Org. Chem. 2014 (2014) 8049 (http://dx.doi.org/10.1002/ejoc.201403123)
D. A. Scudiero, R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, M. R. Boyd, Cancer Res. 48 (1988) 4827 (https://cancerres.aacrjournals.org/content/48/17/4827)
A. Nakayama, H. Sato, S. Nagano, S. Karanjit, H. Imagawa, K. Namba, Chem. Pharm. Bull. 67 (2019) 953 (http://dx.doi.org/10.1248/cpb.c18-00948)
Selectivity Index (SI) is calculated as follows: SI = IC50 of normal cell line (MRC-
-5)/IC50 of cancer cell line. SI values greater than 2 are considered as a satisfactory selectivity (see ref. 14)
M. I. Ahmad, S. Dixit, R. Konwar, P. G. Vasdev, A. K. Yadav, S. Tripathi, M. M. Gupta, A. Sharma, A. Gupta, Bioorg. Med. Chem. Lett. 27 (2017) 5040 (https://doi.org/10.1016/j.bmcl.2017.09.060).