Quantum-mechanical study of the electronic properties of UxPuyOz compounds formed during the recovery of spent nuclear fuel Scientific paper
Main Article Content
Abstract
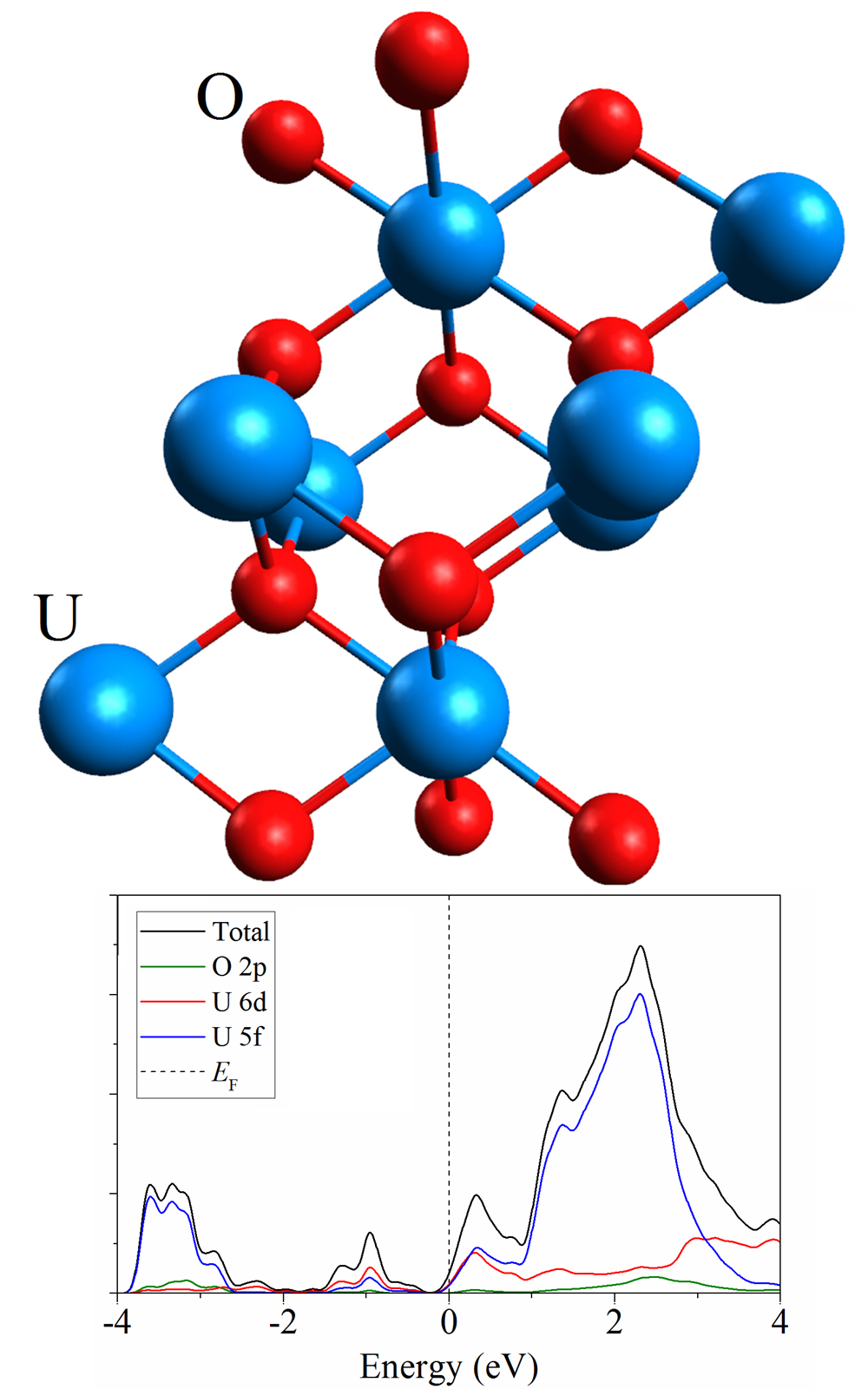
A promising way to recover spent nuclear fuel (SNF) is the method of extracting transuranium compounds from molten salt, which makes it possible to obtain a partial separation between transuranium compounds and lanthanides. This work is devoted to the quantum mechanical study of changes in the structure, energy and electronic properties of the main SNF component, uranium dioxide, upon the removal of oxygen from the system. The influence of the considered properties on the substitution of uranium by plutonium is also studied at a ratio, of the number of plutonium atoms to uranium atoms, of 1:7 and 1:3. The removal of oxygen leads to a narrowing of the band gap up to the transition to a conductive state at a ratio of uranium to oxygen of 2:3. The band gap narrows and metallization sets in even when uranium is replaced by plutonium. A two-stage UO2 metallization scheme based on lithium reduction and direct (electronic) reduction is proposed.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
E. Curti, D.A. Kulik, J. Nucl. Mater. 534 (2020) 152140 (https://doi.org/10.1016/j.jnucmat.2020.152140)
D. H. Hurley, A. El-Azab, M. S. Bryan, M. W. D. Cooper, C. A. Dennett, K. Gofryk, L. He, M. Khafizov, G. H. Lander, M. E. Manley, J. M. Mann, C. A. Marianetti, K. Rickert, F. A. Selim, M. R. Tonks, J. P. Wharry, Chem. Rev. 122 (2022) 3711 (https://doi.org/10.1021/acs.chemrev.1c00262)
C. Ronchi, M. Sheindlin, D. Staicu, M. Kinoshita, J. Nucl. Mater. 327 (2004) 58 (https://doi.org/10.1016/j.jnucmat.2004.01.018)
K. Govers, S. Lemehov, M. How, M. Verwerft, J. Nucl. Mater. 376 (2008) 66 (https://doi.org/10.1016/j.jnucmat.2008.01.023)
S. Kihara, Z. Yoshida, H. Aoiyagi, K. Maeda , O. Shirai , Y. Kitatsuji, Y. Yoshida, Pure Appl. Chem. 71 (1999) 1771 (https://doi.org/10.1351/pac199971091771)
A. Y. Galashev, Int. J. Energy Res. 46 (2022) 3891 (https://doi.org/10.1002/er.7458)
G. L. Fredrickson, T.-S. Yoo, J. Nucl. Mater. 508 (2018) 51 (https://doi.org/10.1016/j.jnucmat.2018.05.037)
A.Y. Galashev, Int. J. Energy Res. 45 (2021) 11459 (https://doi.org/10.1002/er.6267)
T. P. Kaloni, N. Onder, J. Pencer, E. Torres, Ann. Nucl. Energy 144 (2020) 107511 (https://doi.org/10.1016/j.anucene.2020.107511)
J. M. Soler, E. Artacho, J. D. Gale, A. García, J. Junquera, P. Ordejón, D. Sánchez-Portal, J. Phys.: Condens. Matter 14 (2002) 2745 (https://doi.org/10.1088/0953-8984/14/11/302)
S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys, A. P. Sutton, Phys. Rev., B 57 (1998) 1505 (https://doi.org/10.1103/PhysRevB.57.1505)
J. P. Perdew, A. Zunger, Phys. Rev., B 23 (1981) 5048 (https://doi.org/10.1103/PhysRevB.23.5048)
H. J. Monkhorst, J. D. Pack, Phys. Rev., B 13 (1976) 5188 (https://doi.org/10.1103/PhysRevB.13.5188)
C. L. Dugan, G. G. Peterson, A. Mock, C. Young, J. M. Mann, M. Nastasi, M. Schubert, L. Wang, W.-N. Mei, I. Tanabe, P. A. Dowben, J. Petrosky, Eur. Phys. J., B 91 (2018) 67 (https://doi.org/10.1140/epjb/e2018-80489-x)
H. He, D. A. Andersson, D. D. Allred, K. D. Rector, J. Phys. Chem., C 117 (2013) 16540 (https://doi.org/10.1021/jp401149m).