Molecular dynamics simulation of uranium nitride oxidation Scientific paper
Main Article Content
Abstract
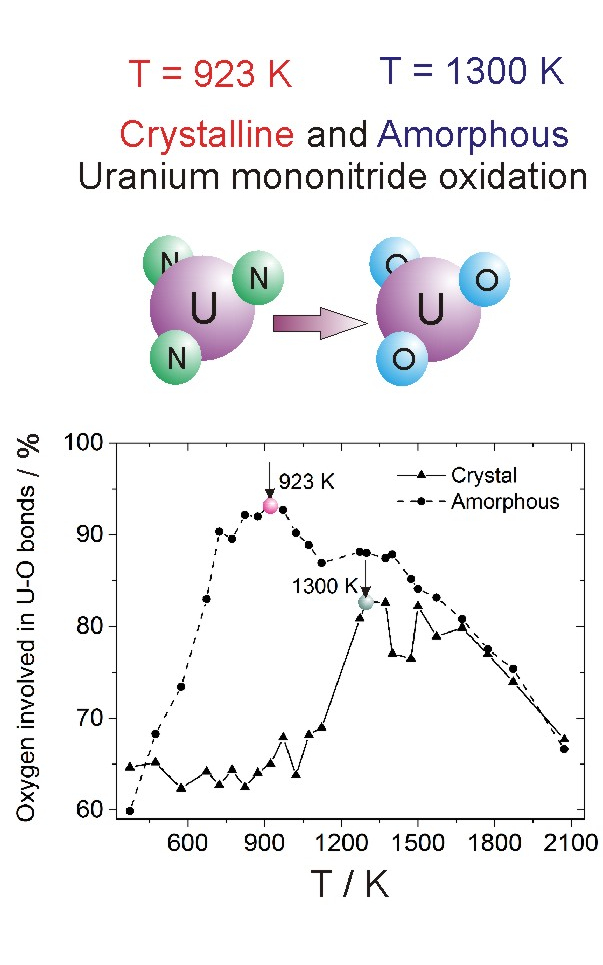
A molecular dynamic simulation of the uranium mononitride (UN) oxidation in an Ar-O medium in the temperature range of 373–2073 K is performed. The study is performed for UN particles with a crystalline and amorphous structure at an oxygen concentration in the gas mixture of 22.5 mol.%. The most efficient oxidation for an amorphous particle occurs at lower temperatures than that for a crystalline particle. Unlike crystalline fragments, amorphous particles undergo more severe fragmentation when they bind to oxygen. Fragmentation of UN particles is one of the main factors regulating the oxidation of finely dispersed media. The oxidation of a UN particle begins from its surface and in the case of an amorphous particle occurs faster than when the particle is crystalline. The process of particle fragmentation is facilitated by the penetration of oxygen atoms inside the particle. An increase in the oxygen concentration in the gas mixture stimulates the oxidation process. The structural changes in the system are investigated by constructing the partial radial distribution functions. The many-body U-N interactions prevent nitrogen escaping into the gaseous environment.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
O. A. Grigorovich, S. V. Abramov, V.I . Voronin, et al. Method of separating nitride nuclear fuel from shell of fuel element fragments. Patent, Ru 2 732 721 С1, 22.09.2020 Bull. № 27.
А. Sajdova, Accident-tolerant uranium nitride. Thesis for the degree of licentiate of engineering. Gothenburg, Sweden 2017 (ISSN: 1652-943X)
C. O. T. Galvin, N. Kuganathan, N. J. Barron, R. W. Grimes, J. Appl. Phys. 135 (2024) 165101 (https://doi.org/10.1063/5.0177315)
L. Yang, N. Kaltsoyannis, J. Nucl. Mater. 577 (2023) 154330 (https://doi.org/10.1016/j.jnucmat.2023.154330)
R. Dell, V. Wheeler, E.J. Mclver, Trans. Faraday Soc. 62 (1966) 3591 (https://doi.org/10.1039/TF9666203591)
E-Y. Choi, C. Y. Won, J-S. Cha, W. Park, H. S. Im, S-S. Hong, J-M. Hur, J. Nucl. Mater. 444 (2014) 261 (https://doi.org/10.1016/j.jnucmat.2013.09.061)
Z. Da-Wei, J. H. Yu, P. Chunying, Y. Song. arXiv:1804.00095 [physics.comp-ph] (https://doi.org/10.48550/arXiv.1804.00095)
P. Tecmer, A. S. P. Gomes, S. Knecht, L. Visscher, J. Chem. Phys. 141 (2014) 041107 (https://doi.org/10.1063/1.4891801)
S. Zhang, F. Wang, J. Phys. Chem. A 121 (2017) 3966 (https://dx.doi.org/10.1021/acs.jpca.7b02985)
G. Liu, C. Zhang, S. M. Ciborowski, A. Asthana, L. Cheng, K. H. Bowen, J. Phys. Chem. A 124 (2020) 6486 (https://dx.doi.org/10.1021/acs.jpca.0c03735)
J. Zhao, C.-X. Chi, L.-Y. Meng, et al., J. Chem. Phys. 157 (2022) 054301 (https://doi.org/10.1063/5.0098068)
A. Sunaga, C. Tabata, T. Yamamura, J. Phys. Chem. A 126 (2022) 8606 (https://doi.org/10.1021/acs.jpca.2c05216)
F. Wei, G. Wu, W.H. Eigen Schwarz, J. Li, Theor. Chem. Acc. 129 (2011) 467 (https://doi.org/110.1007/s00214-010-0885-5)
L. Verlet, Phys. Rev. 159 (1967) 98 (https://doi.org/10.1103/PhysRev.15998)
V. I. Tseplyaev, S. V. Starikov, J. Nucl. Mater. 480 (2016) 7 (https://doi.org/10.1016/j.jnucmat.2016.07.048)
M. W. Cooper, N. Kuganathan, P. A. Burr, M. J.-D. Rusthon, R. W. Grimes, C. R. Stanek, D. A. Andersson, J. Phys.: Condens. Matter. 28 (2016) 405401 (https://doi.org/10.1088/0953-8984/28/40/405401)
M. Krishnamurth, S. Murad, J. D. Olson, Molec. Sim. 32 (2006) 11 (https://doi.org/10.1080/08927020500474318)
K. Kurosaki, K. Yano, K. Yamada, M. Uno, S. Yamanaka, J. Alloys Comp. 311 (2000) 305 (https://doi.org/10.1016/S0925-8388(00)01127-0)
A. Y. Galashev, K. Abramova, A. Vorob’ev, O. Rakhmanova, Yu. Zaikov, Electrochem. Mater. Technol. 2 (2023) 20232017 (https://doi.org/10.15826/elmattech.2023.2.017)
A. Y. Galashev, K. A. Ivanichkina, Yu. P. Zaikov, J. Solid State Chem. 286 (2020) 121278 (https://doi.org/10.1016/j.jssc.2020.121278)
S. Plimpton, J. Comp. Physics 117 (1995) 1. (https://doi.org/10.1006/jcph.1995.1039)
V. G. Baranov, A. V. Tenishev, R. S. Kuzmin, S. A. Pokrovskiy, et al., Ann. Nucl. Energy 87 (2016) 784 (https://doi.org/10.1016/j.anucene.2014.09.023)
S. Zhan, J. Bao, S. Ning, M. Zhang, et al., Chem. Eng. J. 498 (2024) 155322 (https://doi.org/10.1016/j.cej.2024.155322)
R. M. Dell, Y. J. Wheeler, N. J. Bridger, Trans. Faraday Soc. 63 (1967) 1286 (https://doi.org/10.1039/tf9676301286).