Bactericidal effects of copper–polypyrrole composites modified with silver nanoparticles against Gram-positive and Gram-negative bacteria Scientific paper
Main Article Content
Abstract
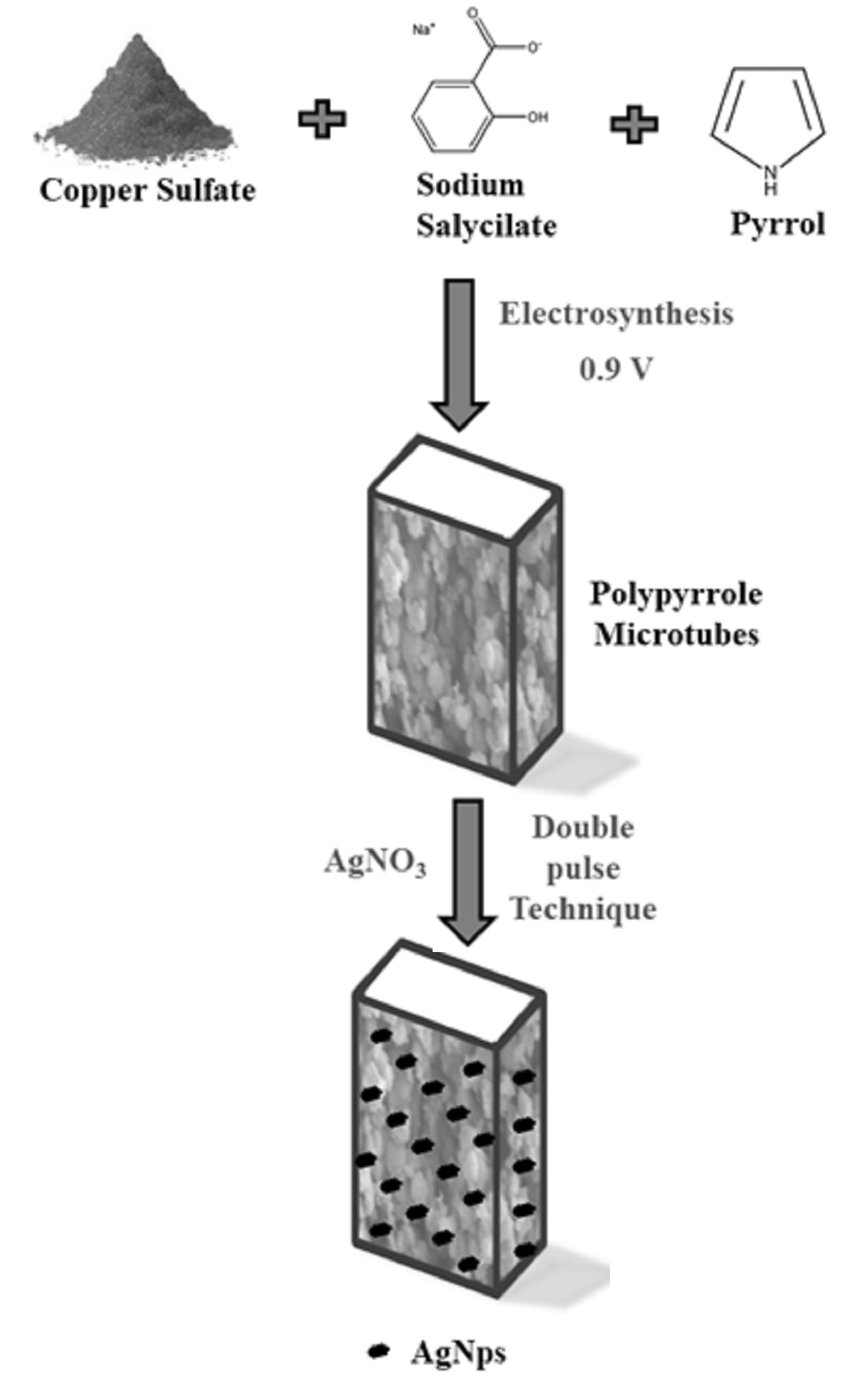
The aim of this research is to study the bactericidal effects of copper–polypyrrole (PPy) composites deposited onto 316L stainless steel (SS) modified with silver nanoparticles (Np). The antimicrobial properties were evaluated against twenty-four strains of Gram-positive and Gram-negative bacteria. Among the twenty-four strains studied, isolates included reference strains (Escherichia coli ATCC 25922, Escherichia coli 0157:H7 EDL 933, Staphylococcus aureus ATCC 25923 and Listeria monocytogenes ATCC 7644), as well as strains isolated from food and clinical samples. The antimicrobial activity of the composites demonstrated that all PPy-modified films had antibacterial properties. Notably, Cu-PPyAgNp500 exhibited the strongest inhibitory activity against both Gram-negative and Gram-positive bacteria. Surface modification of 316L SS with these films is a promising and viable alternative for the development of novel antibacterial composites that can inhibit the growth of a significant number of bacteria.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Consejo Nacional de Ciencia y Tecnología
Grant numbers 2021-2023 GI-11220200102064CO -
Agencia Nacional de Promoción Científica y Tecnológica
Grant numbers PICT-2019-02758 -
Universidad Nacional del Sur
Grant numbers PGI-UNS 24/M159
References
World Health Organization (WHO), 2021, Antimicrobial resistance, Available from https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance аccessed 26 October 2022
World Health Organization (WHO), 2015, Global Action plan antimicrobial resistance, Document Production Services, Geneva, Switzerland. Available from https://ahpsr.who.int/publications/i/item/global-action-plan-on-antimicrobial-resistance аccessed 26 October 2022
E. Seebach, K. F. Kubatzky, Front. Immunol. 10 (2019) 1724 (https://doi.org/10.3389/fimmu.2019.01724)
M. Z. Ibrahim, A. A. D. Sarhan, F. Y. M. Hamdi, J. Alloys Compd. 714 (2017) 636 (https://doi.org/10.1016/j.jallcom.2017.04.231)
N. F. Kamaruzzaman, L. P. Tan, R. H. Hamdan, S. S. Choong, W. K. Wong, A. J. Gibson, A. Chivu, M. de F. Pina, Int. J. Mol. Sci. 20 (2019) 2747 (https://doi.org/10.3390/ijms20112747)
K. Jlassi, M. H. Sliem, F. M. Benslimane, N. O. Eltai, A. M. Abdullah, Prog. Org. Coat. 149 (2020) 105918 (https://doi.org/10.1016/j.porgcoat.2020.105918)
M. Wang, and T. Tang, J. Orthop. Transl. 17 (2019) 42 (https://doi.org/10.1016/j.jot.2018.09.001)
M. Maruthapandi, A. Saravanan, A. Gupta, J. H. T. Luong, A. Gedanken, Macromol. 2 (2022) 78 (https://doi.org/10.3390/macromol2010005)
B. Balasubramaniam, Prateek, S. Ranjan, M. Saraf, P. Kar, S. P. Singh, V. K. Thakur, A. Singh, R. K. Gupta, ACS Pharmacol. Transl. Sci. 4 (2021) 8 (https://doi.org/10.1021/acsptsci.0c00174)
M. Talikowska, X. Fu, G. Lisak, Biosens. Bioelectron. 135 (2019) 50 (https://doi.org/10.1016/j.bios.2019.04.001)
E. N. Zare, T. Agarwal, A. Zarepour, F. Pinelli, A. Zarrabi, F. Rossi, M. Ashrafizadeh, A. Maleki, M. Shahbazi, T. K. Maiti, R. S. Varma, F. R. Tay, M. R. Hamblin, V. Mattoli, P. Makvandi, Appl. Mater. Today 24 (2021) 101117 (https://doi.org/10.1016/j.apmt.2021.101117)
A. Singh, A. Goswami, S. Nain, Appl. Nanosci. 10 (2020) 2255 (https://doi.org/10.1007/s13204-020-01394-y)
M. Balaji, P. Nithya, A. Mayakrishnan, S. Jegatheeswaran, S. Selvam, Y. Cai, J. Yao, M. Sundrarajan, Appl. Surf. Sci. 510 (2020) 145403 (https://doi.org/10.1016/j.apsusc.2020.145403)
M. B. González, D. O. Flamini, L. I. Brugnoni, L. M. Quinzani S. B. Saidman, J. Water Health 16 (2018) 921 (https://doi.org/10.2166/wh.2018.072)
M. B. González, L. I. Brugnoni, D. O. Flamini, L. M. Quinzani, S. B. Saidman, Environ. Monit. Assess. 189 (2017) 53 (https://doi.org/10.1007/s10661-016-5764-7)
M. B. González, L. I. Brugnoni, M. E. Vela, S. B. Saidman, Electrochim. Acta 102 (2013) 66 (https://doi.org/10.1016/j.electacta.2013.03.116)
A. Martinez, L. Brugnoni, D. Flamini, S. Saidman, Prog. Org. Coat. 144 (2020) 105650 https://doi.org/10.1016/j.porgcoat.2020.105650
Y. Qing, L. Cheng, R, Li, G. Liu, Y. Zhang, X. Tang, J. Wang, H. Liu, L. Qin, Int. J. Nanomed. 13 (2018) 3311 (http://dx.doi.org/10.2147/IJN.S165125)
K. M. Rice, G. K. Ginjupalli, N. D. P. K. Manne, C. B. Jones, E. R. Blough, Nanotechnology 30 (2019) 372001 (https://doi.org/10.1088/1361-6528/ab0d38)
T. V. Basova, E. S. Vikulova, S. I. Dorovskikh, A. Hassan, N. B. Morozova, Mater. Des. 204 (2021) 109672 (https://doi.org/10.1016/j.matdes.2021.109672)
D. Mitra, E. T. Kang, K. G. Neoh, Appl. Mater. Interfaces 12 (2020) 21159 (https://doi.org/10.1021/acsami.9b17815)
I. Salah, I. P. Parkin, E. Allan, RSC Adv. 11 (2021) 18179 (https://doi.org/10.1039/D1RA02149D)
Y. Zhuang, S. Zhang, K. Yang, L. Ren, K. Dai, J. Biomed Mater. Res., B 108 (2020) 484 (https://doi.org/10.1002/jbm.b.34405)
P. L. Marucci, N. L. Olivera, L. I. Brugnoni, M. G. Sica, M. A. Cubitto, Environ. Monit. Assess. 175 (2011) 1 (https://doi.org/10.1007/s10661-010-1488-2)
A. Scheludko, M. Todorova, Bull. Acad. Bulg. Sci. Phys. 3 (1952) 61
A. W. Bauer, W. M. Kirby, J. C. Sherris, M. Turck, Am. J. Clin. Pathol. 45 (1966) 493
M. B. González, O. V. Quinzani, M. E. Vela, A. A. Rubert, G. Benítez, S. B. Saidman, Synth. Met. 162 (2012) 1133 (http://doi.org/10.1016/j.synthmet.2012.05.013)
A. Alqudami, S. Annapoorni, P. Sen, R. S. Rawat, Synth. Met. 157 (2007) 53 (http://doi.org/10.1016/j.synthmet.2006.12.006)
M. B. González, S. B. Saidman, Corros. Sci. 53 (2011) 276 (https://doi.org/10.1016/j.corsci.2010.09.021)
R. Holze Polymers 14 (2022) 1584 (https://doi.org/10.3390/polym1408158 4)
Q. Pei, R. Qian, Electrochim. Acta 37 (1992) 1075 (https://doi.org/10.1016/0013-4686(92)85225-A)
A. Singh, Z. Salmi, N. Joshi, P. Jha, A. Kumar, H. Lecoq, S. Lau, M. M. Chehimi, D. K. Aswal, S. K. Gupta, RSC Adv. 3 (2013) 5506 (https://doi.org/10.1039/C3RA22981E)
M. Diantoro, T. Suprayogi, U. Sa’adah, N. Mufti, A. Fuad, A. Hidayat, H. Nur, in Silver Nanoparticles, K. Maaz, Ed., InTech, Rijeka, 2018 (https://doi.org/10.5772/intechopen.75682)
K. F. Babu, P. Dhandapani, S. Maruthamuthu, M. A. Kulandainathan, Carbohydr. Polym. 90 (2012) 1557 (https://doi.org/10.1016/j.carbpol.2012.07.030)
N. Song, S. Chen, D. Tian, Y. Li, C. Wang, X. Lu, Mat. Today Chem. 18 (2020) 100374 (https://doi.org/10.1016/j.mtchem.2020.100374)
J. Shu, Z. Qiu, S. Lv, K. Zhang, D. Tang, Anal. Chem. 89 (2017) 11135 (https://doi.org/10.1021/acs.analchem.7b03491)
B. Chudasama, A. K. Vala, N. Andhariya, R. V. Mehta, R. V. Upadhyay, J. Nanopart. Res. 12 (2010) 1677 (https://doi.org/10.1007/s11051-009-9845-1)
J. S. Kim, E. Kuk, K. N. Yu, J. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C. Hwang, Y. Kim, Y. Lee, D. H. Jeong, M. Cho, Nanomed.: Nanotechnol. Biol. Med. 3 (2007) 95 (https://doi.org/10.1016/j.nano.2006.12.001)
J. Jain, S. Arora, J. M., P. Omray, S. Khandelwal, K. M. Paknikar, Mol. Pharm. 6 (2009)1388 (https://doi.org/10.1021/mp900056g)
J. P. Ruparelia, S. P. Duttagupta, A. K. Chatterjee, S. Mukherji, Acta Biomater. 4 (2008) 707 (https://doi.org/10.1016/j.actbio.2007.11.006)
D. Longano, N.Ditaranto, L. Sabbatini, L. Torsi, N. Cioffi, in Nano-Antimicrobials, N. Cioffi, M. Rai, Eds., Springer, Berlin, 2011, pp. 85–117 (https://doi.org/10.1007/978-3-642-24428-5_3)
G. A. Sotiriou, A. Meye, J. T. Knijnenburg, S. Panke, S. E. Pratsinis, Langmuir 28 (2012) 15929 (https://doi.org/10.1021/la303370d)
J. S. McQuillan, A. M. Shaw, Nanotoxicology 8 (2014) 177 (https://doi.org/10.3109/17435390.2013.870243)
I. P Mukha, A. M. Eremenko, N. P. Smirnova, A. I. Mikhienkova, G. I. Korchak, V. F. Gorchev, A. Y. Chunikhin, Appl. Biochem. Microbiol. 49 (2013) 199 (http://doi.org/10.1134/S0003683813020117)
Y. Dong, H. Zhu, Y. Shen, W. Zhang, L. Zhang, PLoS ONE 14 (2019) e0222322 (https://doi.org/10.1371/journal.pone.0222322)
M. Oves, M. A. Rauf , A. Hussain, H. A. Qari, A. A. P. Khan, P. Muhammad, M. T. Rehman, M. F. Alajmi, I. L. M. Ismail, Front. Pharmacol. 10 (2019) 801 (https://doi.org/10.3389/fphar.2019.00801)
J. R. Morones-Ramirez, J. A. Winkler, C. S. Spina, J. J. Collins. Sci. Transl. Med. 5 (2013) 190ra81 (https://doi.org/10.1126/scitranslmed.3006276.)
S. Khorrami, A. Zarrabi, M. Khaleghi, M. Danaei, M. R. Mozafari, Int. J. Nanomed. 13 (2018) 8013 (https://doi.org/10.2147/IJN.S189295)
G. Applerot, J. Lellouche, A. Lipovsky, Y. Nitzan, R. Lubart, A. Gedanken, E. Banin, Small 8 (2012) 3326 (https://doi.org/10.1002/smll.201200772)
L-U. Rahman, A. Shah, S. K. Lunsford , C. Han, M. N. Nadagoud , E. Sahle-Demessie , R. Qureshi , M. S. Khan, H-B Kraatz, D. D. Dionysiou. RSC Adv. 5 (2015) 44427 (https://doi.org/10.1039/C5RA03633J)
T. V. Basova, E. S. Vikulova, S. I. Dorovskikh, A. Hassan, N. B. Morozova, Mater. Des. 204 (2021) 109672 (https://doi.org/10.1016/j.matdes.2021.109672)
A. Mujeeb, N. A. Khan, F. Jamal, K. F. Badre Alam, H. Saeed, S. Kazmi, A.W.F.Alshameri, M. Kashif, I. Ghazi M. Owais, Front. Chem. 8 (2020) 103 (https://doi.org/10.3389/fchem.2020.00103).