Thermochemistry of pyrolyzed rutin and its esters prepared from facile biocatalytic route Scientific paper
Main Article Content
Abstract
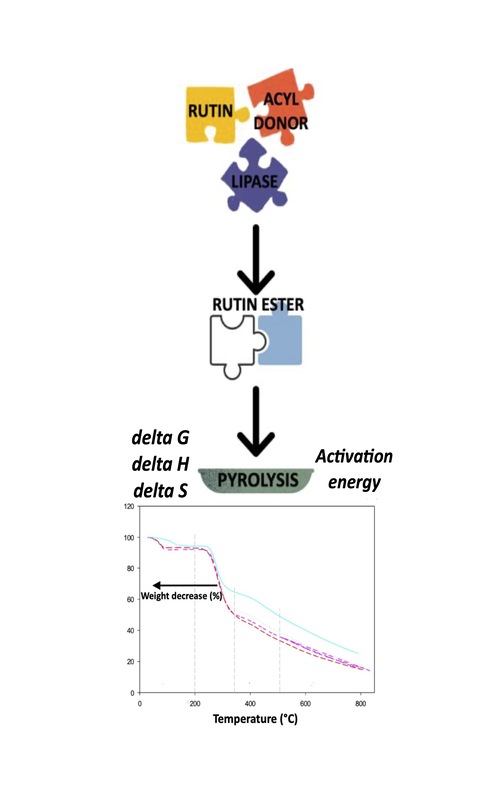
Pyrolysis of quercetin-3-O-rutinoside or rutin and its esters were investigated. Purified ester samples were prepared from lipase-catalyzed esterification of the parent flavonoid, i.e., rutin using acyl donors with different carbon chain length. X-ray diffraction revealed the presence of crystalline peaks in the rutin esters. The degradation activation energies (Ea) as a function of conversion degree a were determined using Kissinger–Akahira–Sunose and Flynn–Wall–Ozawa methods, with corroborative results. Disparity in Ea implies distinct thermal degradation routes. For all studied compounds, degradation is a non-spontaneous process. The presence of acyl moieties and their corresponding carbon chain length in relation to thermodegradation profiles, Ea, entropy (ΔS) and enthalpy (ΔH) changes of the pyrolysis are discussed.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Universiti Malaya
Grant numbers ST072-2021
References
B. Kirschweng, D. M. Tilinger, B. Hégely, G. Samu, D. Tátraaljai, E. Földes, B. Pukánszky, Eur. Polym. J. 103 (2018) 228 (https://doi.org/10.1016/j.eurpolymj.2018.04.016)
A. M. Mahmoud, Exp. Toxicol. Pathol. 64 (2012) 783 (https://doi.org/10.1016/j.etp.2011.01.016)
W. Lee, S. K. Ku, J. S. Bae, Food Chem. Toxicol. 50 (2012) 3048 (https://doi.org/10.1016/j.fct.2012.06.013)
D. S Kim, S. B Lim, Prev. Nutr. Food Sci. 22 (2017) 131 (https://doi.org/10.3746/pnf.2017.22.2.131)
A. Hunyadi, A. Martins, T. J. Hsieh, A. Seres, I. Zupkó, PLoS ONE (2012) (https://doi.org/10.1371/journal.pone.0050619)
J. P. Lin, J. S. Yang, J. J. Lin, K. C. Lai, H. F. Lu, C. Y. Ma, R. S. C. Wu, K. C. Wu, F. S. Chueh, W. G. Wood, J. G. Chung, Environ. Toxicol. 27 (2012) 480 (https://doi.org/10.1002/tox.20662)
R. Mauludin, R. H. Müller, C. M. Keck, Int. J. Pharm. 370 (2009) 202 (https://doi.org/10.1016/j.ijpharm.2008.11.029)
L. Chebil, C. Humeau, A. Falcimaigne, J. M. Engasser, M. Ghoul, Process Biochem. 41 (2006) 2237 (https://doi.org/10.1016/j.procbio.2006.05.027)
J. Viskupicova, M. Ondrejovic, T. Maliar, in Biochemistry, D. Ekinci, Ed., InTech Europe, Rijeka, 2021, p. 263 (https://doi.org/10.5772/34174)
M. E. M. de Araújo, Y. E. Franco, M. C. Messias, G. B. Longato, J. A. Pamphile, P. D. O. Carvalho, Planta Med. 83 (2017) 7 (https://doi.org/10.1055/s-0042-118883)
J. Viskupicova, M . Danihelova, M. Ondrejovic, T. Liptaj, E. Sturdik, Food Chem. 123 (2010) 45 (https://doi.org/10.1016/j.foodchem.2010.03.125)
B. Mbatia, S. S. Kaki, B. Mattiasson, F. Mulaa, P. Adlercreutz, J. Agric. Food Chem. 59 (2011) 7021 (https://doi.org/10.1021/jf200867r)
A. D. M. Sørensen, L. K. Petersen, S. de Diego, N. S. Nielsen, B. M. Lue, Z. Yang, X. Xu, C. Jacobsen, Eur. J. Lipid Sci. Tech. 114 (2012) 434 (https://doi.org/10.1002/ejlt.201100354)
B. M. Lue, N. S. Nielsen, C. Jacobsen, L. Hellgren, Z. Guo, X. Xu, Food Chem. 123 (2010) 221 (https://doi.org/10.1016/j.foodchem.2010.04.009)
J. Viskupicova, M. Majekova, L. Horakova, J. Muscle Res. Cell. M. 36 (2015) 183 (https://doi.org/10.1007/s10974-014-9402-0)
G. Kodelia, K. Athanasiou, F. N. Kolisis, Appl. Biochem. Biotech. 44 (1994) 205 (https://doi.org/10.1007/BF02779657)
F. Mellou, H. Loutrari, H. Stamatis, C. Roussos, F. N. Kolisis, Process Biochem. 41 (2006) 2029 (https://doi.org/10.1016/j.procbio.2006.05.002)
M. I. Cardona, N. M. N. Le, S. Zaichik, D. M. Aragón, A. Bernkop-Schnürch, Int. J. Pharm. 562 (2019) 180 (https://doi.org/10.1016/j.ijpharm.2019.03.036)
N. N. A. Razak, M. S. M. Annuar, Ind. Eng. Chem. Res. 54 (2015) 5604 (https://doi.org/10.1021/acs.iecr.5b00996)
A. Kontogianni, V. Skouridou, V. Sereti, H. Stamatis, F. N. Kolisis, Eur. J. Lipid Sci. Technol. 103 (2010) 655 (https://doi.org/10.1002/1438-9312(200110)103:10<655::AID-EJLT655>3.0.CO;2-X)
M. Ardhaoui, A Falcimaigne, S. Ognier, J. M. Engasser, P. Moussou, G. Pauly, M. Ghoul, J. Biotechnol. 110 (2004) 265 (https://doi.org/10.1016/j.jbiotec.2004.03.003)
M. Şamlı, O. Bayraktar, F. Korel, J. Incl. Phenom. Macromol. 80 (2014) 37 (https://doi.org/10.1007/s10847-014-0396-4)
S. Sun, Y. Jin, Y. Hong, Z. Gu, L. Cheng, L. Zhaofeng, L. Caiming. Food Hydrocoll. 110 (2021) 106224 (https://doi.org/10.1016/j.foodhyd.2020.106224)
H. Chaaban, I. Ioannou, L. Chebil, M. Slimane, C. Gérardin, C. Paris, C. Charbonnel, L. Chekir, M. Ghoul, J. Food Process. Preserv. 41 (2017) e13203 (https://doi.org/10.1111/jfpp.13203)
Ç. Kadakal, T. Duman, R. Ekinci. Food Sci. Technol. 38 (2017) 667-673 (https://doi.org/10.1590/1678-457X.11417)
Ç. Kadakal, T. Duman, Pamukkale Üniversitesi Mühendislik Bilimleri Dergisi 24 (2018) 1370-1375 (https://doi.org/10.5505/pajes.2017.03779)
E. M. da Costa, J. M. Barbosa Filho, T. G. do Nascimento, R. O. Macêdo, Thermochim. Acta 392 (2002) 79 (https://doi.org/10.1016/S0040-6031(02)00087-4)
S. Rohn, N. Buchner, G. Driemel, M. Rauser, L.W. Kroh, J. Agric. Food Chem. 55 (2007) 1568 (https://doi.org/10.1021/jf063221i)
N. Stănciuc, G. Râpeanu, in Non-Alcoholic Beverages, A. Grumezescu, A.M. Holban, Eds., Woodhead Publishing, Sawston, 2019, p. 407 (ISBN 9780128152706)
D. C. de Medeiros, S. S. Mizokami, N. Sfeir, S. R. Georgetti, A. Urbano, R. Casagrande, W. A. Verri, M. M. Baracat, ACS Omega 4 (2019) 1221-1227 (https://doi.org/10.1021/acsomega.8b02868)
M. K. Remanan, F. Zhu, Food Chem. 353 (2021) 128534 (https://doi.org/10.1016/j.foodchem.2020.128534)
M. Turturică, N. Stănciuc, G. Bahrim, G. Râpeanu, Food Bioprocess Tech. 9 (2016) 1706 (https://doi.org/10.1007/s11947-016-1753-7).