New pyrene and fluorene-based π-conjugated Schiff bases: Theoretical and experimental investigation of optical properties Scientific paper
Main Article Content
Abstract
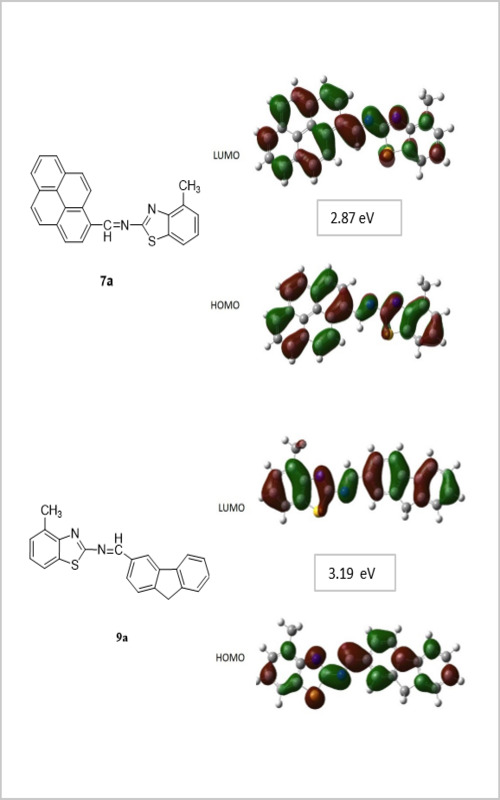
The new Schiff bases with D-π-A system were synthesized by the reaction of polycyclic aldehydes and substituted benzothiazoles. The structures of the synthesized Schiff bases (7a and 9a) were determined by FT-IR, 1H-NMR, 13C-NMR, ESI-Mass and elemental analyses. The optical properties of the new compounds were investigated and the optical band gaps (Eg) were calculated by the Tauc method using the UV–Vis absorption spectra. Density functional theory (DFT/B3LYP/6-31G(d,p)) calculations were conducted to get more insight on the structural and electronic properties of novel Schiff bases. The optimized molecular geometry, UV–Vis spectroscopic parameters and HOMO–LUMO energies were examined and the calculated results were compared with experimental data.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Eskişehir Osmangazi Üniversitesi
Grant numbers 202019059
References
H. Schiff, Justus Liebigs Ann. Chem. 150 (1869) 193
N. Öztürk, MSc Thesis, Istanbul University, 1998
C. Sasaki, K. Nakajima, M. Kojima, J. Fujita, Bull. Chem. Soc. Jpn. 64 (1991) 1318 (https://doi.org/10.1246/bcsj.64.1318)
S. Kanemasa, M. Yoshioka, O. Tsuge, Bull. Chem. Soc. Jpn. 62 (1989) 869 (https://doi.org/10.1246/bcsj.62.869)
M. F. Aly, M. I. Younes, S. A. Metwally, Tetrahedron 50 (1994) 3159 (https://doi.org/10.1016/S0040-4020(01)81114-5)
A. E. Taggi, A. M. Hafez, H. Wack, B. Young, D. Ferraris, T. Lectka, J. Am. Chem. Soc. 124 (2002) 6626 (https://doi.org/10.1021/ja0258226)
K. Singh, M. S. Barwa, P. Tyagi, Eur. J. Med. Chem. 41 (2006) 147 (https://doi.org/10.1016/j.ejmech.2005.06.006)
S. K. Sridhar, M. Saravanan, A. Ramesh, Eur. J. Med. Chem. 36 (2001) 615 (https://doi.org/10.1016/S0223-5234(01)01255-7)
R. Mladenova, M. Ignatova, N. Manolova, T. Petrova, I. Rashkov, Eur. Polym. J. 38 (2002) 989 (https://doi.org/10.1016/S0014-3057(01)00260-9)
M. Koole, R. Frisenda, M. L. Petrus, M. L. Perrin, H. S. van der Zant, T. J. Dingemans, Org. Electron. 34 (2016) 38 (https://doi.org/10.1016/j.orgel.2016.03.043)
N. Bouguerra, A. Růžička, C. Ulbricht, C. Enengl, S. Enengl, V. Pokorná, D. Výprachtický, E. Tordin, R. Aitout, V. Cimrová, D. A. M. Egbe, Macromolecules 49 (2016) 455 (https://doi.org/10.1021/acs.macromol.5b02267)
J. Jankowska, M. F. Rode, J. Sadlej, A. L. Sobolewski, ChemPhysChem 13 (2012) 4287 (https://doi.org/10.1002/cphc.201200560)
K. Haupt, K. Mosbach, Chem. Rev. 100 (2000) 2495 (https://doi.org/10.1021/cr990099w)
S. Pu, Z. Tong, G. Liu, R. Wang, J. Mater. Chem. C 1 (2013) 4726 (https://doi.org/10.1039/C3TC30804A)
M. Petrus, R. Bouwer, U. Lafont, S. Athanasopoulos, N. Greenham, T. Dingemans, J. Mater. Chem. A 2 (2014) 9474 (https://doi.org/10.1039/C4TA01629G)
A. Bolduc, L. Rivier, S. Dufresne, W. Skene, Mater. Chem. Phys. 132 (2012) 722 (https://doi.org/10.1016/j.matchemphys.2011.12.002)
U. H. A. Azeez, D. Ayyappan, S. G. Chidambaram T, R. Singh, J. Subbiah, A. Sambandam, J. Mol. Struct. 1294 (2023) 136315 (https://doi.org/10.1016/j.molstruc.2023.136315)
S. Mukhopadhyay, C. Risko, S. R. Marder, J.-L. Brédas, Chem. Sci. 3 (2012) 3103 (https://doi.org/10.1039/C2SC20861J)
Z. Fatima, H. A. Basha, S. A. Khan, J. Mol. Struct. 1292 (2023) 136062 (https://doi.org/10.1016/j.molstruc.2023.136062)
W. Xu, Z. Shao, Y. Han, W. Wang, Y. Song, H. Hou, Dyes Pigm. 152 (2018) 171179 (https://doi.org/10.1016/j.dyepig.2018.01.056)
G. Turkoglu, M. Cinar, A. Buyruk, E. Tekin, S. Mucur, K. Kaya, T. Ozturk, J. Mater. Chem. C 4 (2016) 6045 (https://doi.org/10.1039/C6TC01285J)
M. Wałęsa‐Chorab, M. H. Tremblay, W. G. Skene, Chem. Eur. J. 22 (2016) 11382 (https://doi.org/10.1002/chem.201600859)
K. S. M. Salih, J. Mol. Struct. 1244 (2021) 131267 (https://doi.org/10.1016/j.molstruc.2021.131267)
K. Mangaiyarkarasi, A. Ravichandran, K. Anitha, A. Manivel, J. Mol. Struct. 1155 (2018) 758 (https://doi.org/10.1016/j.molstruc.2017.11.065)
G. A. Evingür, Ö. Pekcan, Compos. Struct. 183 (2018) 212 (https://doi.org/10.1016/j.compstruct.2017.02.058)
J. Singh, K. Shimakawa, in Advances in Amorphous Semiconductors, J. Singh, K. Shimakawa, Eds., Taylor and Francis New York, 2003 (ISBN 9780415287708)
M. Cossi, N. Rega, G. Scalmani, V. Barone, J. Comput. Chem. 24 (2003) 669 (https://doi.org/10.1002/jcc.10189)
R. Dennington, T. Keith, J. Millam, J.GaussView, version 5, Semichem Inc., Shawnee Mission, KS, 2009
C. Lee, W. Yang, R.G. Parr, Phys. Rev., B 37 (1988) 785 (https://doi.org/10.1103/PhysRevB.37.785)
Gaussian 09, Rev. A, Gaussian Inc, Wallingford, CT, 2009
V. Barone, M. Cossi, J. Phys. Chem., A 102 (1998) 1995. (https://doi.org/10.1021/jp9716997).