Square-pyramidal mononuclear, dinuclear and polymeric copper(II) complexes with (2-pyridinylmethyl)amino derivatives Scientific paper
Main Article Content
Abstract
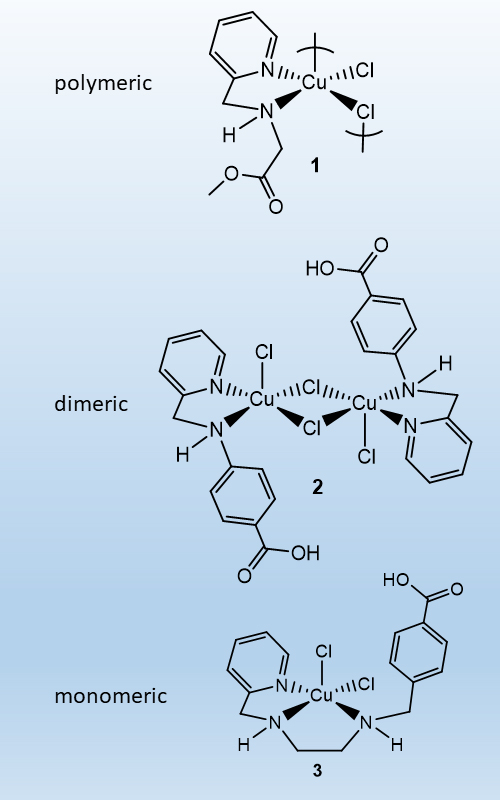
The coordination behavior of three ligand precursors 2-[(2-pyridinylmethyl)amino]acetic acid hydrochloride, 4-[(2-pyridinylmethyl)amino]benzoic acid hydrochloride and 4-{[2-(pyridin-2-ylmethylamino)ethylamino]methyl}benzoic acid hydrochloride, HL1∙HCl–HL3∙HCl, respectively, in copper(II) complexes is described. The complexes were characterized by elemental analysis, ESI mass spectrometry and IR spectroscopy, as well as X-ray structural analysis. The reaction of copper(II) with HL1∙HCl in methanol afforded the polymeric complex [{Cu(µ-Cl)2(MeL1-κ2N,N’)}n] (1) featuring the methyl ester of L1 (MeL1). With HL2∙HCl or HL3∙HCl, the dimeric complex [{CuCl(µ-Cl)(HL2-κ2N,N’)}2] (2) or the mononuclear complex [CuCl2(HL3-κ3N,N’,N’’)] (3) were obtained. All complexes exhibited square-pyramidal geometries. In 1, polymeric chains are formed through bridging chlorido ligands without typical hydrogen bonding interaction. Contrarily, the COOH group in 2 is participating in the formation of intermolecular hydrogen bonding forming a supramolecular structure. In 3, intermolecular hydrogen bonding (Cl···H(O)) leads to a 1-D polymeric structure. The copper(II) complex 2 diminished viability of human 8505C, MCF-7, 518A2 and SW-480 cell lines. The tumoricidal effect of 2 was realized mainly through caspase-mediated apoptosis.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200007
References
M. R. Bond, in Exploring Chemistry with Pyridine Derivatives, IntechOpen, Rijeka, 2022 (https://doi.org/10.5772/intechopen.107124)
R. Diószegi, D. Bonczidai-Kelemen, A. Cs. Bényei, N. V. May, I. Fábián, N. Lihi, Inorg. Chem. 61 (2022) 2319 (https://doi.org/10.1021/acs.inorgchem.1c03728)
T. Klemens, K. Czerwińska, A. Szlapa-Kula, S. Kula, A. Świtlicka, S. Kotowicz, M. Siwy, K. Bednarczyk, S. Krompiec, K. Smolarek, S. Maćkowski, W. Danikiewicz, E. Schab-Balcerzak, B. Machura, Dalton Trans. 46 (2017) 9605 (https://doi.org/10.1039/C7DT01948C)
M. Stojičkov, S. Sturm, B. Čobeljić, A. Pevec, M. Jevtović, A. Scheitler, D. Radanović, L. Senft, I. Turel, K. Andjelković, M. Miehlich, K. Meyer, I. Ivanović-Burmazović, Europ. J. Inorg. Chem. 2020 (2020) 3347 (https://doi.org/10.1002/ejic.202000415)
S. H. Ahn, J. Shin, S. Nayab, H. Lee, Bull. Korean Chem. Soc. 37 (2016) 763 (https://doi.org/10.1002/bkcs.10747)
T. Zhu, Z. Guang-Yi, L. Xue-Qiang, X. Sai-Feng, Z. Qian-Jiang, W. Gregory Jackson, W. Zhan-Bing, L. La-Sheng, Inorg. Chim. Acta 357 (2004) 953 (https://doi.org/10.1016/j.ica.2003.09.027)
A. T. Çolak, O. Z. Yeşilel, O. Büyükgüngör, Polyhedron 29 (2010) 2127 (https://doi.org/10.1016/j.poly.2010.03.024)
M. Shukla, N. Srivastava, S. Saha, T. R. Rao, S. Sunkari, Polyhedron 30 (2011) 754 (https://doi.org/10.1016/j.poly.2010.12.036)
S. J. A. Guieu, A. M. M. Lanfredi, C. Massera, L. D. Pachón, P. Gamez, J. Reedijk, Catal. Today 96 (2004) 259 (https://doi.org/10.1016/j.cattod.2004.06.149)
10. S.-K. Kang, H.-W. Lee, N. Sengottuvelan, Y.-I. Kim, Bull. Korean Chem. Soc. 33 (2012) 95 (https://doi.org/10.5012/bkcs.2012.33.1.95)
A. Mondal, S. Sarkar, D. Chopra, T. N. Guru Row, K. Krishna Rajak, Dalton Trans. (2004) 3244 (https://doi.org/10.1039/B408316D)
X. Wang, J. D. Ranford, J. J. Vittal, J. Mol. Struct. 796 (2006) 28 (https://doi.org/10.1016/j.molstruc.2006.03.090)
X. Wang, J. J. Vittal, Inorg. Chem. 42 (2003) 5135 (https://doi.org/10.1021/ic0344970)
A. M. Alam, M. Nethaji, M. Ray, Angew. Chem. Int. Ed. 42 (2003) 1940 (https://doi.org/10.1002/anie.200250591)
Md. A. Alam, M. Nethaji, M. Ray, Inorg. Chem. 44 (2005) 1302 (https://doi.org/10.1021/ic049145n)
B.-Y. Lou, D.-Q. Yuan, S.-Y. Gao, R.-H. Wang, Y. Xu, L. Han, M.-C. Hong, J. Mol. Struct. 707 (2004) 231 (https://doi.org/10.1016/j.molstruc.2004.07.025)
B.-Y. Lou, Y. Xu, D.-Q. Yuan, L. Han, M.-C. Hong, Acta Cryst. E 60 (2004) m522 (https://doi.org/ 0.1107/S1600536804007342)
B. Sreenivasulu, M. Vetrichelvan, F. Zhao, S. Gao, J. J. Vittal, Eur. J. Inorg. Chem. 2005 (2005) 4635 (https://doi.org/10.1002/ejic.200500638)
Z. Lü, D. Zhang, S. Gao, D. Zhu, Inorg. Chem. Commun. 8 (2005) 746 (https://doi.org/10.1016/j.inoche.2005.05.012)
M. Monroe, Molecular Weight Calculator for Windows, 2011, https://alchemistmatt.com/mwtwin.html.
S. Richter, S. Singh, D. Draca, A. Kate, A. Kumbhar, A. S. Kumbhar, D. Maksimovic-
-Ivanic, S. Mijatovic, P. Lönnecke, E. Hey-Hawkins, Dalton Trans. 45 (2016) 13114 (https://doi.org/10.1039/C6DT01782G)
L. I. Shevchenko, P. S. Pel’kis, M. O. Lozinskii, V. N. Kalinin, Ukr. Khim. Zh. 50 (1984) 301
Rigaku Oxford Diffraction, CrysAlisPro Software system, Rigaku Corporation, Wroclaw, 1995–2023
R. C. Clark, J. S. Reid, Acta Cryst., A 51 (1995) 887 (https://doi.org/10.1107/S0108767395007367)
G. M. Sheldrick, Acta Cryst., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
K. Putz, K. Brandenburg, Diamond Crystal and Molecular Structure Visualization, 2014, https://www.crystalimpact.de/diamond
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, M. R. Boyd, J. Natl. Cancer Inst. 82 (1990) 1107 (https://doi.org/10.1093/jnci/82.13.1107)
V. Vichai, K. Kirtikara, Nat. Protoc. 1 (2006) 1112 (https://doi.org/10.1038/nprot.2006.179)
L. Useini, T. Komazec, M. Laube, P. Lönnecke, J. Schädlich, S. Mijatović, D. Maksimović-Ivanić, J. Pietzsch, E. Hey-Hawkins, Adv. Ther. 6 (2023) 2300117 (https://doi.org/10.1002/adtp.202300117)
I. Predarska, M. Saoud, I. Morgan, P. Lönnecke, G. N. Kaluđerović, E. Hey-Hawkins, Biomolecules 13 (2023) 595 (https://doi.org/10.3390/biom13040595)
Y. Zhang, Z. Bao, N. Lv, K. Chen, C. Zong, H. Yuan, Frontiers Chem. 8 (2020) 609 (https://doi.org/10.3389/fchem.2020.00609)
J. M. Rosenbaum, R. A. Cliff, M. L. Coleman, Anal. Chem. 72 (2000) 2261 (https://doi.org/10.1021/ac991297q)
A. J. Dempster, Nature 136 (1935) 65 (https://doi.org/10.1038/136065b0)
J. B. Creech, J. A. Baker, M. R. Handler, M. Bizzarro, Chem. Geol. 363 (2014) 293 (https://doi.org/10.1016/j.chemgeo.2013.11.009)
N. Pantelić, B. B. Zmejkovski, B. Kolundžija, M. Đ. Crnogorac, J. M. Vujić, B. Dojčinović, S. R. Trifunović, T. P. Stanojković, T. J. Sabo, G. N. Kaluđerović, J. Inorg. Biochem. 172 (2017) 55 (https://doi.org/10.1016/j.jinorgbio.2017.04.001)
G. N. Kaluđerović, H. Schmidt, C. Wagner, K. Merzweiler, D. Steinborn, Collect. Czech. Chem. Commun. 72 (2007) 560 (https://doi.org/10.1135/cccc20070560)
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, G. C. Verschoor, Dalton Trans. (1984) 1349 (https://doi.org/10.1039/DT9840001349)
Y.-F. Liu, D.-F. Rong, H.-T. Xia, D.-Q. Wang, Acta Cryst., E 65 (2009) m1492 (https://doi.org/10.1107/S1600536809044997)
B. Zheng, H. Liu, J. Feng, J. Zhang, Appl. Organometal. Chem. 28 (2014) 372 (https://doi.org/10.1002/aoc.3138)
G. A. Jeffrey, An Introduction to Hydrogen Bonding, UK edition, Oxford University Press, New York, 1997
C. Vetter, C. Wagner, G. N. Kaluderović, R. Paschke, D. Steinborn, Inorg. Chim. Acta 362 (2009) 189 (https://doi.org/10.1016/j.ica.2008.03.085)
R. Lindner, G. N. Kaluđerović, R. Paschkec, C. Wagner, D. Steinborn, Polyhedron 27 (2008) 914 (https://doi.org/10.1016/j.poly.2007.11.020)
G. N. Kaluđerović, T. Krajnović, M. Momčilović, S. Stosic-Grujicic, S. Mijatović, D. Maksimović-Ivanić, E. Hey-Hawkins, J. Inorg. Biochem. 153 (2015) 315 (https://doi.org/10.1016/j.jinorgbio.2015.09.006)
X.-H. Yang, T. L. Sladek, X. Liu, B. R. Butler, C. J. Froelich, A. D. Thor, Cancer Res. 61 (2001) 348 (https://aacrjournals.org/cancerres/article/61/1/348/507421/Reconstitution-of-Caspase-3-Sensitizes-MCF-7?searchresult=1)
R. U. Jänicke, M. L. Sprengart, M. R. Wati, A. G. Porter, J. Biol. Chem. 273 (1998) 9357 (https://doi.org/10.1074/jbc.273.16.9357)
R. Uauy, M. Olivares, M. Gonzalez, Am. J. Clin. Nutr. 67 (1998) 952S (https://doi.org/10.1093/ajcn/67.5.952S)
V. C. Shanbhag, N. Gudekar, K. Jasmer, C. Papageorgiou, K. Singh, M. J. Petris, Biochim. Biophys. Acta Mol. Cell Res. 1868 (2021) 118893 (https://doi.org/10.1016/j.bbamcr.2020.118893).