The influence of polyvinyl alcohol concentration toward conductivity and permeability of chitosan–montmorillonite composite membrane Scientific paper
Main Article Content
Abstract
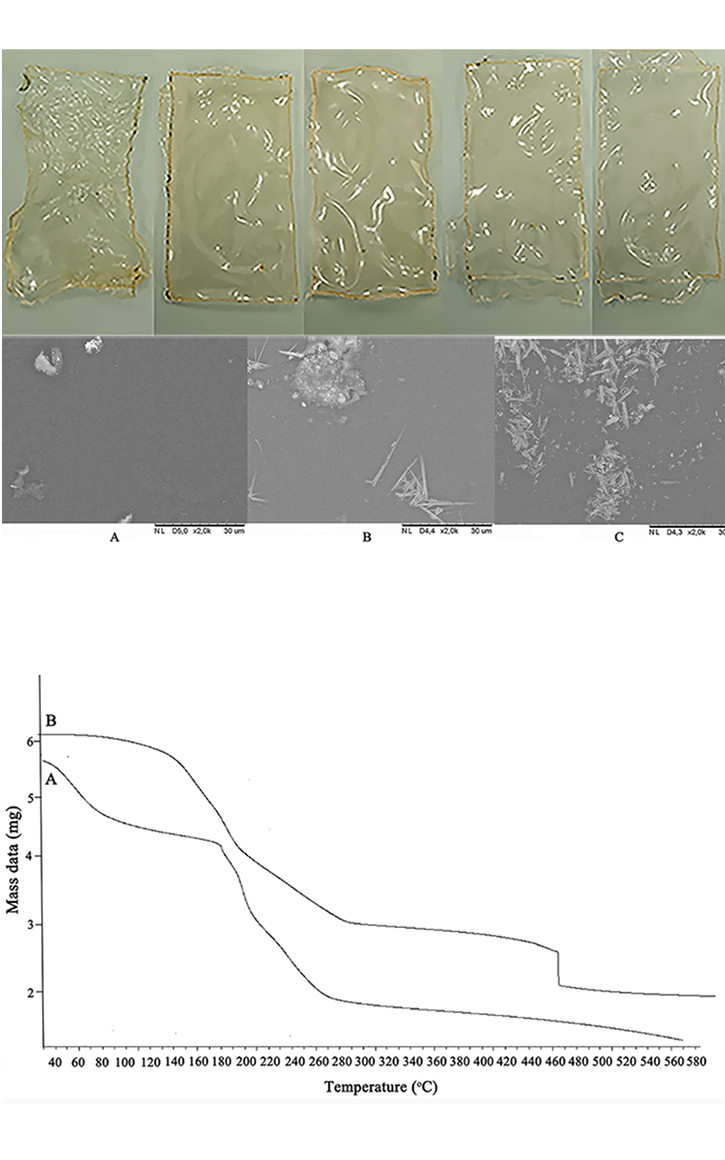
The composite membrane is synthesized using chitosan as a matrix membrane with montmorillonite (MMT) as a filler and modified using polyvinyl alcohol (PVA). The main aim of this study is to find out the influence of PVA concentration and the working temperature toward the permeability of chitosan–MMT/PVA composite membrane. Fourier transform infrared spectroscopy (FTIR) characterization is performed in order to identify the interaction between the chitosan matrix and the modified MMT with PVA. The presence of new absorption at 1116.82 and 619.17 cm-1 indicated the interaction between MMT and PVA. Further, the widening of OH absorption indicated the hydrogen bond which is formed between chitosan matrix and PVA. This interaction is also demonstrated by the evenly distributed surface on scanning electron microscope (SEM) topography analysis. The thermal stability of composite membrane is determined by thermal gravimetry analysis (TGA). In addition, the composite membrane containing PVA has four patterns decomposed. When the TVA is absent from the composite membrane, it has three decomposition patterns, which are shown by TGA analysis. Based on its tensile strength, the composite membrane has good mechanical properties. The proton conductivity of the composite membranes are directly proportional to the PVA concentration. On the other hand, the methanol permeability of composite membranes is inversely proportional with the PVA concentration. The highest proton conductivity was obtained with the addition of 2 % PVA of 2.94×10-4 S cm-1. Further, it also has the lowest methanol permeability with the value of 5.05×10-6 cm2 s-1. As a result, the crosslinked composite membrane chitosan–MMT prepared by PVA-crosslinking technique has the potential to be exploited for the direct methanol fuel cell application.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
K. Sopian, W. R. Wan Daud, Renew. Energy 31 (2006) 719 (http://dx.doi.org/10.1016/j.renene.2005.09.003)
V. Neburchilov, J. Martin, H. Wang, J. Zhang, J. Power Sources 169 (2007) 221 (http://dx.doi.org/10.1016/j.jpowsour.2007.03.044)
N. Shaari, S. K. Kamarudin, J. Power Sources 289 (2015) 71 (http://dx.doi.org/10.1016/j.jpowsour.2015.04.027)
S. Pricl, P. Posocco, G. Scocchi, M. Fermeglia, Handb. Nanophysics Funct. Nanomater. (2010) 3-1-3 (http://dx.doi.org/10.4325/seikeikakou.25.110)
P. G. Allison, R. D. Moser, M. Q. Chandler, J. A. Caminero-Rodriguez, K. Torres-
-Cancel, O. G. Rivera, J. R. Goodwin, E. R. Gore, C. A. Weiss, J. Nanomater. 2015 (2015) (http://dx.doi.org/10.1155/2015/291248)
M. Monroy-Barreto, J. C. Aguilar, E. Rodríguez de San Miguel, A. L. Ocampo, M. Muñoz, J. de Gyves, J. Memb. Sci. 344 (2009) 92 (http://dx.doi.org/10.1016/j.memsci.2009.07.039)
T. Fu, Z. Cui, S. Zhong, Y. Shi, C. Zhao, G. Zhang, K. Shao, H. Na, W. Xing, J. Power Sources 185 (2008) 32 (http://dx.doi.org/10.1016/j.jpowsour.2008.07.004)
A. Rahmatulloh, L. Atmadja, J. Serbian Chem. Soc. 86 (2021) 831 (http://dx.doi.org/10.2298/JSC201118043R)
Y. F. Yang, G. S. Gai, Z. F. Cai, Q. R. Chen, J. Hazard. Mater. 133 (2006) 276 (http://dx.doi.org/10.1016/j.jhazmat.2005.10.028)
G. Paradossi, R. Lisi, M. Paci, V. Crescenzi, J. Polym. Sci., A 34 (1996) 3417 (http://dx.doi.org/10.1002/pola.1996.874)
H. Lin, C. Zhao, W. Ma, K. Shao, H. Li, Y. Zhang, H. Na, J. Power Sources 195 (2010) 762 (http://dx.doi.org/10.1016/j.jpowsour.2009.08.020)
M. A. Abu-Saied, E. A. Soliman, E. A. A. Desouki, Mater. Today Commun. 25 (2020) 101536 (http://dx.doi.org/10.1016/j.mtcomm.2020.101536)
K. Hari Gopi, V. M. Dhavale, S. D. Bhat, Mater. Sci. Energy Technol. 2 (2019) 194 (http://dx.doi.org/10.1016/j.mset.2019.01.010)
M. Purwanto, L. Atmaja, M. T. Salleh, M. A. Mohamed, J. Jaafar, A. F. Ismail, M. Santoso, N. Widiastuti, Malaysian J. Anal. Sci. 21 (2017) 675 (http://dx.doi.org/10.17576/mjas-2017-2103-17)
J. Zawadzki, H. Kaczmarek, Carbohydr. Polym. 80 (2010) 394 (http://dx.doi.org/10.1016/j.carbpol.2009.11.037)
C. H. Chen, F. Y. Wang, C. F. Mao, W. T. Liao, C. D. Hsieh, Int. J. Biol. Macromol. 43 (2008) 37 (http://dx.doi.org/10.1016/j.ijbiomac.2007.09.005)
P. B. Palani, K. S. Abidin, R. Kannan, M. Sivakumar, F. M. Wang, S. Rajashabala, G. Velraj, RSC Adv. 4 (2014) 61781 (http://dx.doi.org/10.1039/c4ra10788h)
V. Vijayalekshmi, D. Khastgir, J. Memb. Sci. 523 (2017) 45 (http://dx.doi.org/10.1016/j.memsci.2016.09.058)
P. Pei, M. Wang, D. Chen, P. Ren, L. Zhang, Prog. Nat. Sci. Mater. Int. 30 (2020) 751 (http://dx.doi.org/10.1016/j.pnsc.2020.08.015)
J. Maiti, N. Kakati, S. H. Lee, S. H. Jee, B. Viswanathan, Y. S. Yoon, J. Power Sources 216 (2012) 48 (http://dx.doi.org/10.1016/j.jpowsour.2012.05.057)
T. Li, Y. Yang, J. Power Sources 187 (2009) 332 (http://dx.doi.org/10.1016/j.jpowsour.2008.11.035)
H. Wu, B. Zheng, X. Zheng, J. Wang, W. Yuan, Z. Jiang, J. Power Sources 173 (2007) 842 (http://dx.doi.org/10.1016/j.jpowsour.2007.08.020)
S. N. Ayyubi, L. Admaja, Walisongo J. Chem. 3 (2020) 1 (http://dx.doi.org/10.21580/wjc.v3i1.6018).