Ultrasonic and spectroscopic investigations of molecular interactions in binary mixture of PEG-400 and DMSO at different temperatures Scientific paper
Main Article Content
Abstract
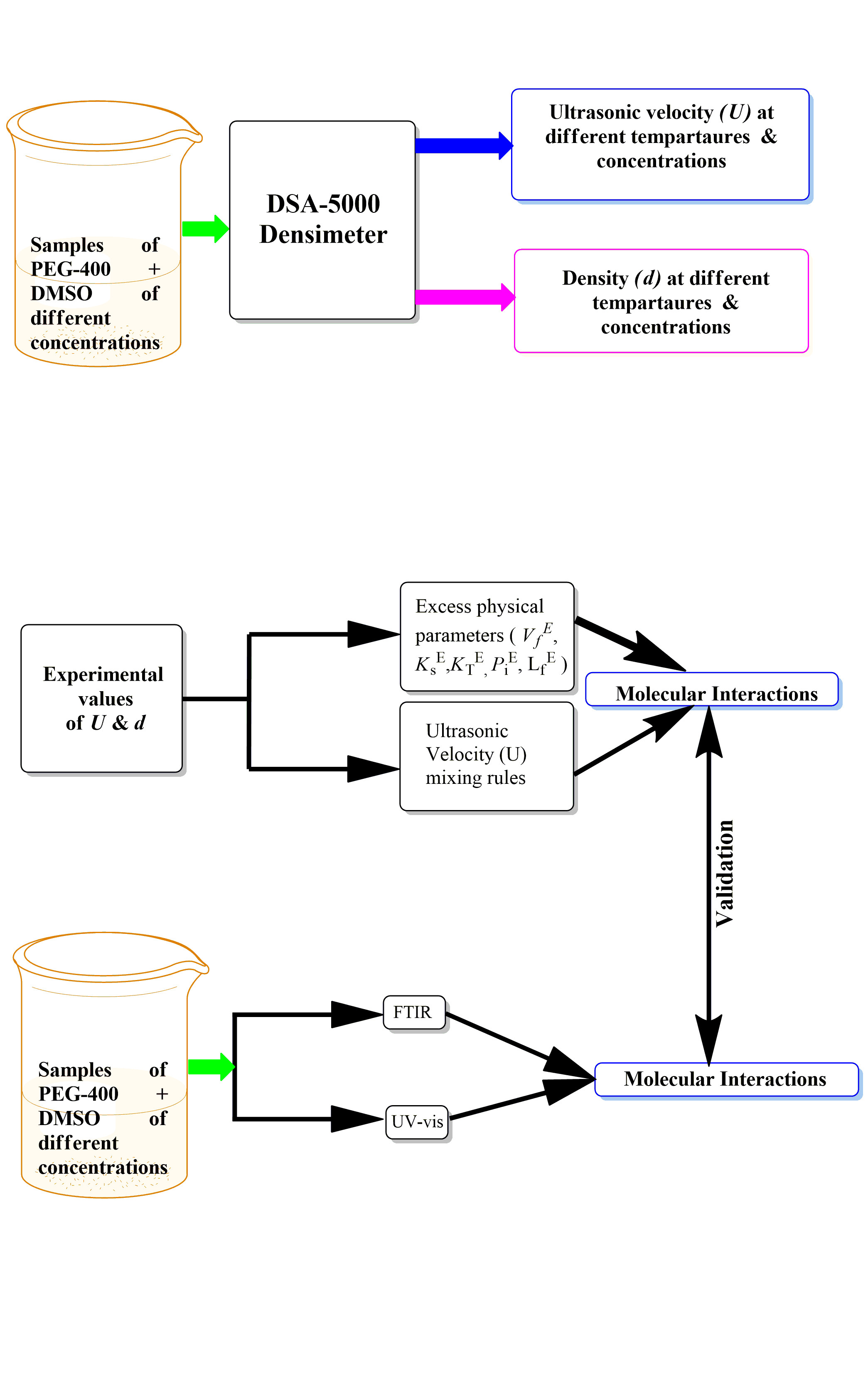
In the present study, the ultrasonic velocity and density data for the binary mixture of polyethylene glycol (PEG)-400 and dimethyl sulfoxide (DMSO), at various concentrations and different temperatures (T, 288.15, 298.15 and 308.15 K), have been measured and further apllied to determine several physical parameters such as adiabatic and isothermal compressibility, intermolecular free length, internal pressure and free volume. The excess values of these parameters have also been computed and fitted with the Redlich–Kister (R–K) polynomial equation. The nature, type, and strength of intermolecular interactions present within the PEG-400 + DMSO mixture have been explained based on the sign and magnitude of excess values. Furthermore, partial molar volumes, excess partial molar volumes, apparent molar volumes and apparent molar volumes at infinite dilution have also been determined to investigate the solute–solvent interactions. Various mixing rules such as the ideal mixing rule (Uim), Nomoto relation (UN), impedance dependence relation (UZ), Junjie relation (UJ), van Deal–Vangeel relation (UV) and collision factor theory (UCFT) are employed to compute the ultrasonic velocity and compared with the experimental one. Among these relations, the Nomoto and Junjie relations are found to be most suitable for the given mixture. In addition to it, the present system has also been examined using Fourier transform infra-red (FTIR) and UV–Vis spectroscopic techniques. The change in intensity and shift in peak position in the FTIR and UV–Vis spectra of the PEG-400 + DMSO mixture are used to confirm the intermolecular hydrogen bonding in the given system.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
P. Malik, G. Chauhan, P. Kumar, A. Deep, Liq. Cryst. 49 (2022) 2008 (https://doi.org/10.1080/02678292.2022.2094006)
P. Kumar, V. Sharma, P. Malik, K. K. Raina, J. Mol. Struct. 1196 (2019) 866 (https://doi.org/10.1016/j.molstruc.2019.06.045)
S. Tian, Y. Hou, W. Wu, S. Ren, J. Qian, J. Hazard. Mater. 278 (2014) 409 (https://doi.org/10.1016/j.jhazmat.2014.06.037)
K. T. Lee, A. R. Mohamed, S. Bhatia, K. H. Chu, J. Chem. Eng. 114 (2005) 171 (https://doi.org/10.1016/j.cej.2005.08.020)
T. Zhao, J. Zhang, B. Guo, F. Zhang, F. Sha, X. Xie, X. Wei, J. Mol. Liq. 207 (2015) 315 (https://doi.org/10.1016/j.molliq.2015.04.001)
K. Zhang, J. Yang, X. Yu, J. Zhang, X. Wei, J. Chem. Eng. Data 56 (2011) 3083 (https://doi.org/10.1021/je200148u)
J. Rodríguez-Sevilla, M. Álvarez, G. Limiñana, M. C. Díaz, J. Chem. Eng. Data 47 (2002) 1339 (https://doi.org/10.1021/je015538e)
D. Nagel, R. de Kermadec, H. G. Lintz, C. Roizard, F. Lapicque, Chem. Eng. Sci. 57 (2002) 4883 (https://doi.org/10.1016/S0009-2509(02)00283-X)
F. Han, J. Zhang, G. Chen, X. Wei, J. Chem. Eng. Data 53 (2008) 2598 (https://doi.org/10.1021/je800464t)
S. Trivedi, C. Bhanot, S. Pandey, J. Chem. Thermodyn. 42 (2010) 1367 (https://doi.org/10.1016/j.jct.2010.06.001)
A. Upmanyu, M. Dhiman, D. P. Singh, H. Kumar, J. Mol. Liq. 334 (2021) 115939 (https://doi.org/10.1016/j.molliq.2021.115939)
B. V. Kumar Naidu, K. C. Rao, M. C. S. Subha, J. Chem. Eng. Data 47 (2002) 379 (https://doi.org/10.1021/je0101395)
M. Dhiman, K. Singh, J. Kaushal, A. Upmanyu, D. P. Singh, Acta Acust. United Acust. 105 (2019) 743 (https://doi.org/10.3813/AAA.919354)
M. Rani, S. Gahlyan, A. Gaur, S. Maken, Chin. J. Chem. Eng. 23 (2015) 689 (https://doi.org/10.1016/j.cjche.2014.12.003)
P. Kaur, N. Chakraborty, K. C. Juglan, H. Kumar, J. Mol. Liq. 315 (2020) 113763 (https://doi.org/10.1016/j.molliq.2020.113763)
D. P. Gupta, A. Upmanyu, M. Dhiman, D. P. Singh, Estimation of ultrasonic velocity and viscosity of polymer solutions of HTPB+ chlorobenzene at different temperatures, AIP Conf. Proc., AIP Publishing, Melville, NY, 2022 (https://doi.org/10.1063/5.0080974)
A. Ali, A. K. Nain, V. K. Sharma, S. Ahmad, Phys. Chem. Liq. 42 (2004) 375 (https://doi.org/10.1080/00319100410001679882)
M. Umadevi, R. Kesavasamy, K. Rathina, R. Mahalakshmi. J. Mol. Liq. 219 (2016) 820 (https://doi.org/10.1016/j.molliq.2016.03.085)
L. Ma, F. Sha, X. Qiao, Q. Li, J. Zhang, Chin. J. Chem. Eng. 25 (2017) 1249 (https://doi.org/10.1016/j.cjche.2017.01.001)
G. Arivazhagan, A. Elangovan, R. Shanmugam, R. Vijayalakshmi, N. K. Karthick, J. Mol. Liq. 214 (2016) 357 (https://doi.org/10.1016/j.molliq.2015.10.062)
L. M. Madikizela, P. S. Mdluli, L. Chimuka, React. Funct. Polym. 103 (2016) 33 (https://doi.org/10.1016/j.reactfunctpolym.2016.03.017)
T. S. Krishna, K. Raju, M. Gowrisankar, A. K. Nain, B. Munibhadrayya, J. Mol. Liq. 216 (2016) 484 (https://doi.org/10.1016/j.molliq.2016.01.085)
A. Awasthi, J. P. Shukla, Ultrasonics 41 (2003) 477 (https://doi.org/10.1016/S0041-624X(03)00127-6)
P. Malik, S. Kumar, Khushboo, A. Upmanyu, P. Kumar, P. Malik, Liq. Cryst. 49 (2022) 1604 (https://doi.org/10.1080/02678292.2022.2102684)
O. E.-A. A. Adam, A. A. Hassan, Phys. Chem. Liq. 56 (2018) 55 (https://doi.org/10.1080/00319104.2017.1292424)
N. Chaudhary, A. Kumar Nain, Phys. Chem. Liq. 58 (2020) 736 (https://doi.org/10.1080/00319104.2019.1636378)
M. T. Zafarani-Moattar, N. Tohidifar, J. Chem. Eng. Data 51 (2006) 1769 (https://doi.org/10.1021/je0601715)
M. T. Zafarani-Moattar, N. Tohidifar, J. Chem. Eng. Data 53 (2008) 785 (https://doi.org/10.1021/je700651e)
H. Patel, Z. S. Vaid, U. U. More, S. P. Ijardar, N. I. Malek, J. Chem. Thermodyn. 99 (2016) 40 (https://doi.org/10.1016/j.jct.2016.02.025)
F. M. Sannaningannavar, B. S. Navati, N. H. Ayachit, J. Therm. Anal. Calorim. 112 (2013) 1573 (https://doi.org/10.1007/s10973-012-2724-5)
J. G. Baragi, M. I. Aralaguppi, T. M. Aminabhavi, M. Y. Kariduraganavar, A. S. Kittur, J. Chem. Eng. Data 50 (2005) 910 (https://doi.org/10.1021/je049610v)
S. Baluja, R. M. Talaviya, J. Chem. Eng. Data 61 (2016) 1431 (https://doi.org/10.1021/acs.jced.5b00627)
V. K. Syal, S. Chauhan, R. Gautam, Ultrason. 36 (1998) 619 (https://doi.org/10.1016/S0041-624X(97)00104-2)
D. Keshapolla, R. L. Gardas, Fluid Phase Equilib. 383 (2014) 32 (https://doi.org/10.1016/j.fluid.2014.09.022)
T. Zhao, Q. Xu, J. Xiao, X. Wei, J. Chem. Eng. Data 60 (2015) 2135 (https://doi.org/10.1021/acs.jced.5b00209)
A. Ali, F. Nabi, J. Dispers. Sci. Technol. 31 (2010) 1326 (https://doi.org/10.1080/01932690903227469)
H. Wang, W. Liu, J. Huang, J. Chem. Thermodyn. 36 (2004) 743 (https://doi.org/10.1016/j.jct.2004.04.004)
R. K. Shukla, S. N. Dixit, P. Jain, P. Mishra, S. Sharma, Orbital-Electron. J. Chem. 2 (2011) 356 (https://periodicos.ufms.br/index.php/orbital/article/view/17980/12479)
J. C. De La Torre, Ann. N. Y. Acad. Sci. 411 (1983) 293 (https://doi.org/10.1111/j.1749-6632.1983.tb47311.x)
M. T. Zafarani-Moattar, H. Shekaari, J. Chem. Thermodyn. 38 (2006) 624 (https://doi.org/10.1016/j.jct.2005.07.018)
A. Ali, A. K. Nain, D. Chand, R. Ahmad, Bull. Chem. Soc. Jpn. 79 (2006) 702 (https://doi.org/10.1246/bcsj.79.702)
J. Krakowiak, D. Bobicz, W. Grzybkowski, J. Mol. Liq. 88 (2000) 197 (https://doi.org/10.1016/S0167-7322(00)00154-9)
H. Shekaari, M. T. Zafarani-Moattar, Int. J. Thermophys. 29 (2008) 534 (https://doi.org/10.1007/s10765-008-0395-z)
S. R. Dandwate, S. B. Deshmukh, J. Curr. Pharma Res. 10 (2020) 3716 (ISSN-2230-7842 CODEN-CPRUE6, www.jcpronline.in/)
U. R. Kapadi, S. K. Chavan, O. S. Yemul, J. Chem. Eng. Data 42 (1997) 548 (https://doi.org/10.1021/je960216+)
G. Ritzoulis, Can. J. Chem. 67 (1989) 1105 (https://doi.org/10.1139/v89-166)
M. A. Saleh, O. Ahmed, M. S. Ahmed, J. Mol. Liq. 115 (2004) 41 (https://doi.org/10.1016/j.molliq.2003.12.021)
S. B. Aznarez, L. Mussari, M. A. Postigo, J. Chem. Eng. Data 38 (1993) 270 (https://doi.org/10.1021/je00010a022)
S. Parveen, D. Shukla, S. Singh, K. P. Singh, M. Gupta, J. P. Shukla, Appl. Acoust. 70 (2009) 507 (https://doi.org/10.1016/j.apacoust.2008.05.008)
B. Nagarjun, A. V. Sarma, G. R. Rao, C. Rambabu, J. Thermodyn. 2013 (2013) 285796 (https://doi.org/10.1155/2013/285796)
A. Zhu, J. Wang, R. Liu, J. Chem. Thermodyn. 43 (2011) 796 (https://doi.org/10.1016/j.jct.2010.12.027)
A. Ali, A. K. Nain, V. K. Sharma, S. Ahmad, Phys. Chem. Liq. 42 (2004) 375 (https://doi.org/10.1080/00319100410001679882)
P. R. Bevington, D. K. Robinson, Data reduction and error analysis for the physical sciences, McGraw-Hill, New York, 1969, pp. 235–242 (ISBN 0-07-247227-8)
M. Dhiman, K. Singh, D. P. Gupta, D. P. Singh, A. Upmanyu, Study of excess acoustical and thermo-dynamical parameters of binary solutions of polypropylene glycol-400 and
n-alkanols at 303 K, AIP Conf. Proc., AIP Publishing, Melville, NY, 2020 (https://doi.org/10.1063/5.0001107)
B. Thanuja, G. Nithya, C. C. Kanagam, Ultrason. Sonochem. 19 (2012) 1213 (https://doi.org/10.1016/j.ultsonch.2012.03.006)
A. Awasthi, A. Awasthi, Phys. Chem. Liq. 51 (2013) 112 (https://doi.org/10.1080/00319104.2012.690569)
G. V Rao, A. V. Sarma, D. Ramachandran, C. Rambabu, Ind. J. Pure App. Phys. 42 (2004) 820 (http://nopr.niscpr.res.in/handle/123456789/8851)
S. F. Babavali, D. Punyaseshudu, K. Narendra, C. S. Yesaswi, C. Srinivasu, J. Mol. Liq. 224 (2016) 47 (https://doi.org/10.1016/j.molliq.2016.09.079)
M. Dhiman, A. Upmanyu, P. Kumar, D. P. Singh, Karbala Int. J. Mod. Sci. 8 (2022) 703 (https://doi.org/10.33640/2405-609X.3270)
S. Gahlyan, M. Rani, S. Maken, J. Mol. Liq. 199 (2014) 42 (https://doi.org/10.1016/j.molliq.2014.08.011)
H. E. Hoga, R. B. Torres, J. Chem. Thermodyn. 43 (2011) 1104 (https://doi.org/10.1016/j.jct.2011.02.018).