Immobilization of periodate-oxidized horseradish peroxidase by adsorption on sepiolite Scientific paper
Main Article Content
Abstract
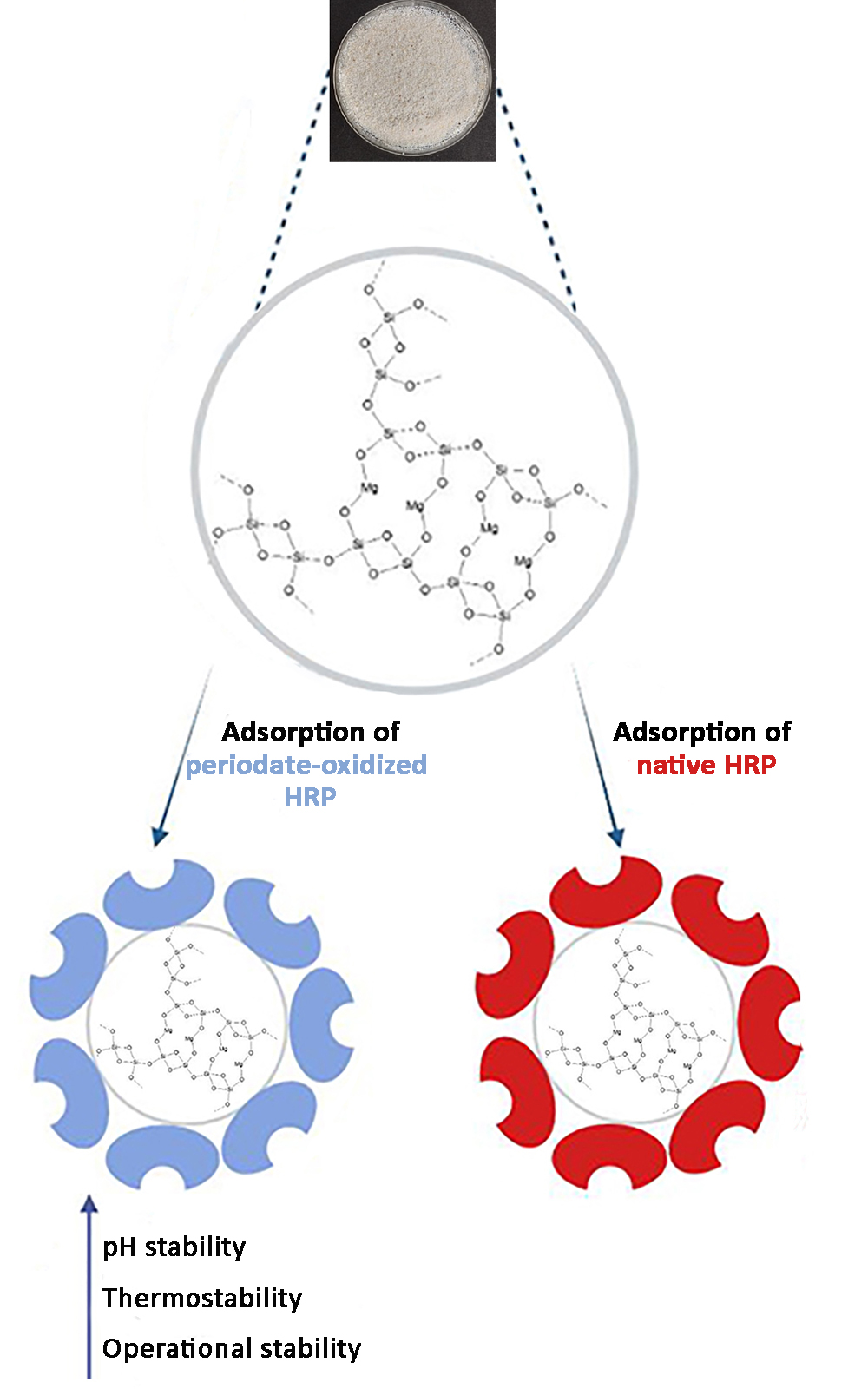
Horseradish peroxidases (HRP), native and periodate-oxidized were immobilized onto sepiolite clay mineral by adsorption. Both peroxidases were adsorbed on this carrier in different quantities. Specific activity of immobilized enzymes was increased with increasing the amount of peroxidase added per gram of sepiolite. The highest specific activity was achieved when 15 mg of peroxidase was added per g of sepiolite. Also, periodate-oxidized enzymes showed similar specific activity as native ones. Stability studies (pH, thermal and operational stability) were conducted for both peroxidases. Residual specific activity of HRP immobilized onto sepiolite declined with an increase of incubation time at 65 °C. Oxidized-peroxidase lost 64 % of the initial activity, whereas native HRP dropped 92 % of its activity after 5 min of incubation at 65 °C. Reduction of the enzyme activity was observed with the temperature increase from 30 to 80 °C. pH profiles of native peroxidase immobilized onto sepiolite were higher in both acidic and basic regions compared to periodate-oxidized enzyme. Oxidized HRP was more successful in studies of operational stability, it retained 42 % of its activity after 4 consecutive cycles of pyrogallol oxidation, whereas native peroxidase kept only 11 % of the original activity.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200168;451-03-47/2023-14/200288;451-03-47/2023-01/200053
References
C. L. B. Reis, E. Y. A. de Sousa, J. de F. Serpa, R. C. Oliveira, J. C. S. dos Santos, Quim. Nova 42 (2019) 768. (https://doi.org/10.21577/0100-4042.20170381)
S. Cantone, V. Ferrario, L. Corici, C. Ebert, D. Fattor, P. Spizzo, L. Gardossi, Chem. Soc. Rev. 42 (2013) 6262 (https://doi.org/10.1039/C3CS35464D)
D. M. Liu, J. Chen, Y. P. Shi, TrAC – Trends Anal. Chem. 102 (2018) 332 (https://doi.org/10.1016/j.trac.2018.03.011)
C. Garcia-Galan, Á. Berenguer-Murcia, R. Fernandez-Lafuente, R. C. Rodrigues, Adv. Synth. Catal. 353 (2011) 2885 (https://doi.org/10.1002/adsc.201100534)
Homaei, R. Sariri, F. Vianello, R. Stevanato, J. Chem. Biol. 6 (2013) 185 (http://dx.doi.org/10.1007/s12154-013-0102-9)
Brena, P. González-Pombo, F. Batista-Viera, Immobilization of Enzymes and Cells: 3rd ed., Methods in Molecular Biology 1051 (2013) 15 (https://doi.org/10.1007/978-1-62703-550-7_2)
Z. Ashkan, R. Hemmati, A. Homaei, A. Dinari, M. Jamlidoost, A. Tashakor, Int. J. Biol. Macromol. 168 (2020) 708 (https://doi.org/10.1016/j.ijbiomac.2020.11.127)
Homaei, in Advances in Food Biotechnology, R. V. Rai, Ed., John Wiley & Sons, Hoboken, NJ, 2015, p. 145 (https://doi.org/10.1002/9781118864463.ch09)
R. Ismail, K. H. Baek, Int. J. Biol. Macromol. 163 (2020) 1624 (https://doi.org/10.1016/j.ijbiomac.2020.09.021)
F. Largo, R. Haounati, S. Akhouairi, H. Ouachtak, R. El Haouti, A. El Guerdaoui, N. Hafid, D. M. F. Santos, F. Akbal, A. Kuleyin, A Jada, A. A. Addi, J. Mol. Liq. 318 (2020) 114247 (https://doi.org/10.1016/j.molliq.2020.114247)
Y. Olshansky, S. Masaphy, R. A. Root, G. Rytwo, Appl. Clay Sci. 152 (2018) 143 (https://doi.org/10.1016/j.clay.2017.11.006)
M. S. Carrasco, J. C. Rad, S. Gonzalez-Carcedo, Biores. Technol. 51 (1995) 175 (https://doi.org/10.1016/0960-8524(94)00115-H)
M. E. Sedaghat, M. Ghiaci, H. Aghaei, S. Soleimanian-Zad, Appl. Clay Sci. 46 (2009) 131 (https://doi.org/10.1016/j.clay.2009.07.021)
M. Shirvani, B. Khalili, M. Kalbasi, H. Shariatmadari, F. Nourbakhsh, Clays Clay Miner. 68 (2020) 287 (https://doi.org/10.1007/s42860-020-00066-w)
S. Cengiz, L. Çavaş, K. Yurdakoç, Appl. Clay. Sci. 65–66 (2012) 114 (https://doi.org/10.1016/j.clay.2012.06.004)
S. Mortazavi, H. Aghaei, Int. J. Biol. Macromol. 164 (2020) 1 (https://doi.org/10.1016/j.ijbiomac.2020.07.103)
N. Caza, J. K. Bewtra, N. Biswas, K. E. Taylor, Water Res. 33 (1999) 3012 (https://doi.org/10.1016/S0043-1354(98)00525-9)
F. Quintanilla-Guerrero, M. A. Duarte-Vázquez, B. E. García-Almendarez, R. Tinoco, R. Vazquez-Duhalt, C. Regalado, Biores. Technol. 99 (2008) 8605 (https://doi.org/10.1016/j.biortech.2008.04.031)
Alemzadeh, S. Nejati, J. Haz. Mat. 166 (2009) 1082 (https://doi.org/10.1016/j.jhazmat.2008.12.026)
N. Miletić, A. Nastasović, K. Loos, Biores. Technol. 115 (2012) 126 (https://doi.org/10.1016/j.biortech.2011.11.054)
Z. Knezevic, N. Milosavic, D. Bezbradica, Biochem. Eng. J. 30 (2006) 269 (https://doi.org/10.1016/j.bej.2006.05.009)
R. M. Prodanović, M. B. Simić, Z. M. Vujčić, J. Serb. Chem. Soc. 68 (2003) 819 (https://doi.org/10.2298/JSC0311819P)
N. Milosavić, R. Prodanović, S. Jovanović, Z. Vujčić, Enzyme Microb. Technol. 40 (2007) 1422 (https://doi.org/10.1016/j.enzmictec.2006.10.018)
R. Prodanović, S. Jovanović, Z. Vujčić, Biotechnol. Lett. 23 (2001) 1171 (https://doi.org/10.1023/A:1010560911400)
H. Öztürk, E. Pollet, V. Phalip, Y. Güvenilir, L. Avérous, Polymers 8 (2016) 416 (https://doi.org/10.3390/polym8120416)
H. N. Oztop, C. Hepokur, D. Saraydin, J. Food Sci. 74 (2009) N45 (https://doi.org/10.1111/j.1750-3841.2009.01302.x)
S. F. Torabi, K. Khajeh, S. Ghasempur, N. Ghaemi, S. O. R. Siadat, J. Biotechnol. 131 (2007) 111 (https://doi.org/10.1016/j.jbiotec.2007.04.015)
H. J. Kim, Y. Suma, S. H. Lee, J. A. Kim, H. S. Kim, J. Mol. Catal., B 83 (2012) 8 (https://doi.org/10.1016/j.molcatb.2012.06.012)
L. Zhang, C. Gu, J. Xiong, M. Yang, Y. Guo, Sci. China Chem. 58 (2015) 731 (https://doi.org/10.1007/s11426-014-5196-6)
M. Kurosawa, T. Itoh, Y. Kodera, A. Matsushima, M. Hiroto, H. Nishimura, Y. Inada, Bioconjugate Chem. 13 (2002) 167 (https://doi.org/10.1021/bc000133+)
J. Xiong, C. Hang, J. Gao, Y. Guo, C. Gu, Chem. Eng. J. 254 (2014) 276 (https://doi.org/10.1016/j.cej.2014.05.139)
K. Chattopadhyay, S. Mazumdar, Biochemistry 39 (2000) 263 (https://doi.org/10.1021/bi990729o)
N. Ž. Šekuljica, N. Ž. Prlainović, J. R. Jovanović, A. B. Stefanović, V. R. Djokić, D. Ž. Mijin, Z. D. Knežević-Jugović, Bioprocess Biosyst. Eng. 39 (2016) 461 (https://doi.org/10.1007/s00449-015-1529-x)
W. Liu, W. C. Wang, H. S. Li, X. Zhou, Water Sci. Tech. 63 (2011) 1621 (https://doi.org/10.2166/wst.2011.228)
N. Pantić, M. Spasojević, Ž. Stojanović, Đ. Veljović, J. Krstić, A. M. Balaž, R. Prodanović, O. Prodanović, J. Polym. Environ. 30 (2022) 3005 (https://doi.org/10.1007/s10924-021-02364-3)
S. Mortazavi, H. Aghaei, Int. J. Biol. Macromol. 164 (2020) 1 (https://doi.org/10.1016/j.ijbiomac.2020.07.103).