Dispersive liquid–liquid microextraction for determining urinary muconic acid as benzene biological indicator Scientific paper
Main Article Content
Abstract
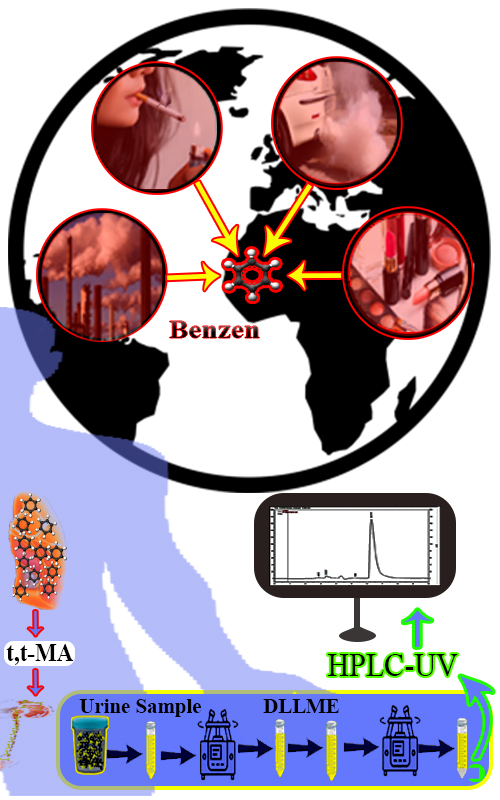
The monitoring of occupational exposure to chemicals is essential for assessing the workplace. In the case of hazardous and carcinogenic chemicals, such as benzene, occupational monitoring becomes even more crucial. Trans,trans-muconic acid (t,t-MA) is one of the benzene urinary metabolites. Pretreatment methods for t,t-MA generally include liquid–liquid extraction and solid–phase extraction. Using dispersive liquid–liquid microextraction (DLLME) during sample preparation and extraction can reduce extraction costs and environmental impacts. Furthermore, the process is cost-effective and easy to operate. This study is aimed to develop, optimize, and validate an analytical method for measuring t,t-MA concentration in urine matrix through DLLME combined with high-performance liquid chromatography. In this method, five variables including pH, the volume of the extractant and the disperser, salt content and the time of centrifugation were optimized using the response surface methodology with a central composite design approach and experimental data. The proposed DLLME was successfully applied to real samples of exposed workers to benzene with extraction efficiencies from 95.8 to 102.4 %. The optimum conditions were pH 8, extractant solvent, 300 µL, disperser solvent, 300 µL, salt, 3.4 % and centrifuge, 3 min. According to the result of this study, the proposed DLLME approach can be effectively applied to the biomonitoring of individuals exposed to benzene.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
References
C. Carvalhais, M. Querido, C. C. Pereira, J. Santos, Work 69 (2021) 3 (https://doi.org/10.3233/WOR-205302)
L. De Maria, C. Ledda, A. Caputi, F. Mansi, E. S. S. Cannone, S. Sponselli, D. Cavone, F. Birtolo, E. Cannizzaro, G. M. Ferri, V. Rapisarda, L. Vimercati, Front. Public Heal. 8 (2020) 271 (https://doi.org/10.3389/fpubh.2020.00271)
Y. R. Li, C. J. Xie, C. X. Qiu, Q. Y. Lin, Y. M. Liu, Chin. J. Ind. Hygiene Occupat. Dis. 37 (2019) 119 (https://doi.org/10.3760/CMA.J.ISSN.1001-9391.2019.02.007)
S. B. Wilbur, S. M. S. Keith, O. Faroon, D. Wohlers, ATSDR’s Toxicol. Profiles (2002) (https://doi.org/10.1201/9781420061888_ch38)
N. Mirzaei, K. Naddafi, Raminnabizadeh, K. Yaghmaeian, M. S. Assanvand, S. Maroufizadeh, M. Hoseini, S. Adabi, M. Yunesian, Acta Medica Mediterr. 32 (2016) 1471 (https://www.actamedicamediterranea.com/archive/2016/special-issue-4/urinary-benzene-as-a-biomarker-of-environmental-exposure-to-benzene-in-males-in-the-general-population/document)
T. Tunsaringkarn, J. Suwansaksri, S. Soogarun, W. Siriwong, A. Rungsiyothin, K. Zapuang, M. Robson, Asian Pacific J. Cancer Prev. 12 (2011) 223 (https://journal.waocp.org/article_25501_cd29634a0ebf3dad97742bb9edc1e432.pdf)
E. A. Natelson, Am. J. Hematol. 82 (2007) 826 (https://doi.org/10.1002/ajh.20934)
M. Borgie, A. Garat, F. Cazier, A. Delbende, D. Allorge, F. Ledoux, D. Courcot, P. Shirali, Z. Dagher, Chemosphere 96 (2014) 122 (https://doi.org/10.1016/j.chemosphere.2013.09.034)
S. M. Rappaport, S. Kim, Q. Lan, R. Vermeulen, S. Waidyanatha, L. Zhang, G. Li, S. Yin, R. B. Hayes, N. Rothman, M. T. Smith, Environ. Health Perspect. 117 (2009) 946 (https://doi.org/10.1289/ehp.0800510)
E. Soleimani, Rev. Anal. Chem. 39 (2020) 168 (https://doi.org/10.1515/revac-2020-0116)
S. Viegas, M. Z. Jeddi, N. B. Hopf, J. Bessems, N. Palmen, K. S. Galea, K. Jones, P. Kujath, R. C. Duca, H. Verhagen, T. Santonen, R. Pasanen-Kase, Int. J. Environ. Res. Public Health 17 (2020) 5884 (https://doi.org/10.3390/ijerph17165884)
I. Pilia, M. Campagna, G. Marcias, D. Fabbri, F. Meloni, G. Spatari, D. Cottica, C. Cocheo, E. Grignani, F. De-Giorgio, P. Cocco, E. D’aloja, Int. J. Environ. Res. Public Health 18 (2021) 4644 (https://doi.org/10.3390/ijerph18094644)
F. Ghamari, A. Bahrami, Y. Yamini, F. G. Shahna, A. Moghimbeigi, Anal. Chem. Insights 2016 (2016) 65 (https://doi.org/10.4137/Aci.s40177)
Threshold Limit Values (TLVs®) and Biological Exposure Indices (BEIs®), 2022, https://www.techstreet.com/standards/2022-threshold-limit-values-tlvs-and-biological-exposure-indices-beis?product_id=2242171#jumps (accessed 1 January, 2022)
F. Dehghani, F. Omidi, O. Heravizadeh, S. Yousefinejad, Sci. Rep. 11 (2021) 15751 (https://doi.org/10.1038/s41598-021-95174-5)
A. Damokhi, S. Yousefinejad, S. Jafari, E. Soleimani, F. Dehghani, J. Mol. Liq. 386 (2023) 122506 (https://doi.org/10.1016/j.molliq.2023.122506)
D. Basu, S. Bag, S. Mukherjee, G. Goel, J. Acad. Clin. Microbiol. 21 (2019) 66 (https://doi.org/10.4103/jacm.jacm_20_18)
A. Barbieri, L. Sabatini, A. Accorsi, A. Roda, F. S. Violante, Rapid Commun. Mass Spectrom. 18 (2004) 1983 (https://doi.org/10.1002/rcm.1580)
S. Abbaszadeh, S. Yousefinejad, S. Jafari, E. Soleimani, J. Sep. Sci. 44 (2021) 3126 (https://doi.org/10.1002/jssc.202100044)
M. Tehranirokh, M. Van den Bronk, P. Smith, Z. Dai, K. Ragunathan, A. Muscalu, S. Mills, M. C. Breadmore, R. A. Shellie, J. Chromatogr., A 1642 (2021) 462032 (https://doi.org/10.1016/j.chroma.2021.462032)
M. Rezaee, Y. Yamini, S. Shariati, A. Esrafili, M. Shamsipur, J. Chromatogr., A 1216 (2009) 1511 (https://doi.org/10.1016/j.chroma.2008.12.091)
M. Rismanchian, K. Ebrahim, Z. Ordudari, Chem. Pap. 73 (2019) 2485 (https://doi.org/10.1007/s11696-019-00800-2)
S. Yousefinejad, F. Honarasa, N. Saeed, J. Sep. Sci. 38 (2015) 1771 (https://doi.org/10.1002/jssc.201401427)
M. Nekoeinia, S. Yousefinejad, A. Abdollahi-Dezaki, Ind. Eng. Chem. Res. 54 (2015) 12682 (https://doi.org/10.1021/acs.iecr.5b02982)
S. Yousefinejad, F. Honarasa, H. Montaseri, RSC Adv. 5 (2015) 42266 (https://doi.org/10.1039/c5ra05930e).