Application of magnetite nanoparticle-modified walnut shell as an adsorbent for the removal of the organic dye Coomassie Brilliant Blue R-250 Scientific paper
Main Article Content
Abstract
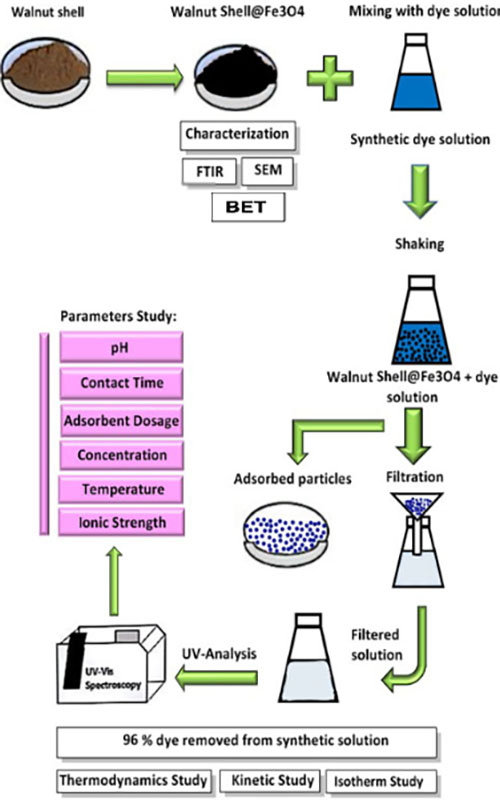
In this research, a magnetic nanocomposite, walnut shell@Fe3O4, was synthesized as a natural adsorbent for the removal of Coomassie Brilliant Blue (CBB) R-250 organic dye from aqueous solutions, achieving a remarkable removal efficiency of 96.16 %. The morphology of the nanocomposite was characterized using SEM and FTIR, revealing particle sizes of less than 18 nm. Additionally, BET analysis was performed, indicating a high surface area that enhances adsorption capacity. The influential variables affecting dye removal, including solution pH, stirring time, adsorbent dosage, initial dye concentration, temperature and ionic strength, were optimized. The adsorption process was analysed using Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherm models. The experimental results indicated that the process followed the Freundlich and Temkin isotherm models, suggesting the heterogeneous nature of the adsorbent surface. The kinetic conditions of adsorption were investigated using pseudo-first order and pseudo-second-order models, with results showing that the adsorption process of CBB followed the pseudo-second-order kinetic model, indicating the chemical adsorption of the dye onto the magnetic nanoparticles. The thermodynamic studies also revealed the spontaneous nature of the adsorption process, with a positive slope of the Van’t Hoff curve indicating an exothermic reaction. Due to the equilibrium time of 5 min in the adsorption mechanism, the synthesized magnetic nanocomposite demonstrated a high CBB dye removal rate, making it suitable for treating dye-containing solutions.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
References
R. Zein, H. Fathony, P. Ramadhani, Deswati, J. Serb. Chem. Soc. 89 (2024) 123 (https://doi.org/10.2298/JSC230303084Z)
M. Vukčević, M. Maletić, B. Pejić, N. Karić, K. Trivunac, A. Perić Grujić, J. Serb. Chem. Soc. 88 (2023) 669 (https://doi.org/10.2298/JSC221213015V)
Z. Y. Velkova, G. K. Kirova, M. S. Stoytcheva, V. Gochev, J. Serb. Chem. Soc. 83 (2018) 107 (https://doi.org/10.2298/JSC170519093V)
N. Nourbakhsh, H. Zavvar Mousavi, E. Kolvari, A. Khaligh, Appl. Chem. Today 17 (2023) 33 (https://doi.org/10.22075/chem.2021.23896.1991)
M. Ghereghlou, A. A. Esmaeili, M. Darroudi, Sep. Sci. Technol. 57 (2022) 2005 (https://doi.org/10.1080/01496395.2022.2029490)
V. K. Veni, T. H. Brenda, IOP Conf. Ser.: Earth Environ. Sci. 765 (2021) 012039 (https://doi.org/10.1088/1755-1315/765/1/012039)
B. M. Thamer, A. Aldalbahi, M. Moydeen A, H. El-Hamshary, A. M. Al-Enizi, M. H. El-Newehy, Mater. Chem. Phys. 234 (2019) 133 (https://doi.org/10.1016/j.matchemphys.2019.05.087)
G. Sharma, M. Naushad, A. Kumar, S. Rana, S. Sharma, A. Bhatnagar, F. J. Stadler, A. A. Ghfar, M. R. Khan, Process Saf. Environ. Prot. 109 (2017) 301 (https://doi.org/10.1016/j.psep.2017.04.011)
J. A. Putri, A. Suratman, R. Roto, J. Metastable Nanocrystall. Mater. 34 (2022) 63 (https://doi.org/10.4028/v-4f958r)
A. O. Ezzat, A. M. Tawfeek, J. R. Rajabathar, H. A. Al-Lohedan, Molecules 27 (2022) 441 (https://www.mdpi.com/1420-3049/27/2/441)
A. O. Ezzat, A. M. Tawfeek, F. Mohammad, H. A. Al-Lohedan, J. Mol. Liq. 358 (2022) 119195 (https://doi.org/10.1016/j.molliq.2022.119195)
S. Dhananasekaran, R. Palanivel, S. Pappu, J. Adv. Res. 7 (2016) 113 (https://doi.org/10.1016/j.jare.2015.03.003)
P. F. de Sales, Z. M. Magriotis, M. A. L. S. Rossi, R. F. Resende, C. A. Nunes, J. Environ. Manage. 130 (2013) 417 (https://doi.org/10.1016/j.jenvman.2013.08.067)
G. R. Chaudhary, P. Saharan, A. Umar, S. K. Mehta, S. Mor, Sci. Adv. Mater. 5 (2013) 1886 (https://doi.org/10.1166/sam.2013.1701)
M. Altikatoglu, M. Celebi, Artif. Cells Blood Substit. Biotechnol. 39 (2011) 185 (https://doi.org/10.3109/10731199.2010.533124)
N. T. Abdel-GhANi, G. A. El-Chaghaby, E.-S. A. Rawash, E. C. Lima, J. Chil. Chem. Soc. 62 (2017) 3505 (https://doi.org/10.4067/S0717-97072017000200016)
S. Dehghan Abkenar, M. Hassannezhad, M. Hosseini, M. R. Ganjali, J. Serb. Chem. Soc. 84 (2019) 701 (https://doi.org/10.2298/JSC181228038D)
X.-S. Li, G.-T. Zhu, Y.-B. Luo, B.-F. Yuan, Y.-Q. Feng, TrAC, Trends Anal. Chem. 45 (2013) 233 (https://doi.org/10.1016/j.trac.2012.10.015)
M. Bordbar, N. Negahdar, B. Khodadadi, J. Nanostruct. 12 (2022) 262 (https://doi.org/10.22052/JNS.2022.02.005)
S. Rahnama, S. Shariati, F. Divsar, Comb. Chem. High Through. Screen. 21 (2018) 583 (https://doi.org/10.2174/1386207321666181019111211)
M. Khan, S. Naseer, M. Khan, R. Nazir, A. Badshah, Adnan, S. Shujah, A. Parveen, Desal. Water Treat. 228 (2021) 286 (https://doi.org/10.5004/dwt.2021.27352)
A. Ali Ahmed, Z. Hattab, Y. Berredjem, S. Hamoudi, R. Djellabi, Desal. Water Treat. 317 (2024) 100278 (https://doi.org/10.1016/j.dwt.2024.100278)
M. Erfani, R. Ansari, H. Zavvar Mousavi, Appl. Chem. Today 17 (2022) 149 (https://doi.org/10.22075/chem.2022.23980.1994)
S. Temel, E. Yaman, N. Ozbay, F. O. Gokmen, J. Serb. Chem. Soc. 85 (2020) 939 (https://doi.org/10.2298/JSC190517114T)
D. Kosale, C. Thakur, V. K. Singh, J. Serb. Chem. Soc. 88 (2023) 653 (https://doi.org/10.2298/JSC220830021K)
S. Jadali, S. M. Sajjadi, H. Zavvar Mousavi, M. Rajabi, Anal. Bioanal. Chem. Res. 4 (2017) 171 (https://doi.org/10.22036/abcr.2016.67517.1122)
M. Hadnađev Kostić, T. Vulić, Đ. Karanović, M. Milanović, J. Serb. Chem. Soc. 87 (2022) 1011 (https://doi.org/10.2298/JSC220228034H)
Z. Lotfi, H. Z. Mousavi, S. M. Sajjadi, Anal. Methods 9 (2017) 4504 (https://doi.org/10.1039/C7AY01166K)
J. Rahchamani, H. Z. Mousavi, M. Behzad, Desalination 267 (2011) 256 (https://doi.org/10.1016/j.desal.2010.09.036)
S. M. Seyed Danesh, H. Faghihian, S. Shariati, J. Nano Res. 52 (2018) 54 (https://doi.org/10.4028/www.scientific.net/JNanoR.52.54)
Z. Dahaghin, H. Z. Mousavi, L. Boutorabi, J. Mol. Liq. 243 (2017) 380 (https://doi.org/10.1016/j.molliq.2017.08.018)
S. Eftekhari, M. R. Sohrabi, S. Mortazavinik, Iran. J. Chem. Chem. Eng. (2024) (https://doi.org/10.30492/ijcce.2024.2016928.6343)
D. Marković, S. Milovanović, M. Radoičić, Ž. Radovanović, I. Zizovic, Z. Šaponjić, M. Radetić, J. Serb. Chem. Soc. 83 (2018) 1379 (https://doi.org/10.2298/JSC180913089M)
C. A. P. Almeida, N. A. Debacher, A. J. Downs, L. Cottet, C. A. D. Mello, J. Colloid Interface Sci. 332 (2009) 46 (https://doi.org/10.1016/j.jcis.2008.12.012)
I. Espinoza, C. Sandoval Pauker, L. Ramos Guerrero, P. Vargas Jentzsch, F. Muñoz Bisesti, J. Serb. Chem. Soc. 85 (2020) 547 (https://doi.org/10.2298/JSC190804119E)
A. A. Fodeke, O. O. Olayera, J. Serb. Chem. Soc. 84 (2019) 1143 (https://doi.org/10.2298/JSC190209042F)
S. K. Hassaninejad-Darzi, H. Z. Mousavi, M. Ebrahimpour, J. Mol. Liq. 248 (2017) 990 (https://doi.org/10.1016/j.molliq.2017.10.126)
K. Gul, H. Khan, N. Muhammad, B. Ara, T. U. H. Zia, Sep. Sci. Technol. 56 (2021) 2507 (https://doi.org/10.1080/01496395.2020.1839498)
A. C. Enache, P. Samoila, C. Cojocaru, R. Apolzan, G. Predeanu, V. Harabagiu, Sustainability 15 (2023) 2704 (https://doi.org/10.3390/su15032704)
I. Badran, R. Khalaf, Sep. Sci. Technol. 55 (2020) 2433 (https://doi.org/10.1080/01496395.2019.1634731).