The role of non-covalent interactions in the solvation dynamics of metronidazole in water: A theoretical study Scientific paper
Main Article Content
Abstract
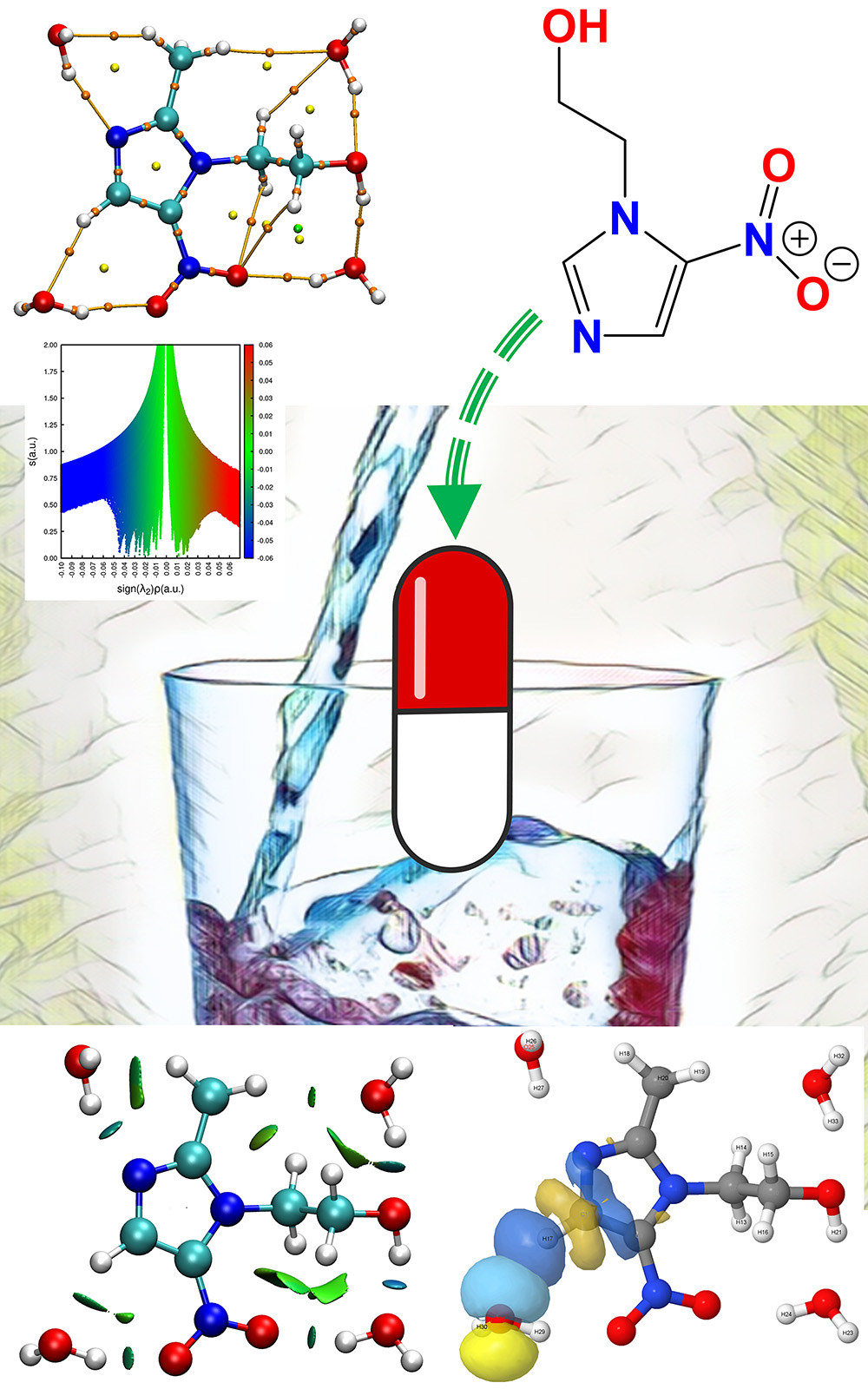
Metronidazole, the medicine with the brand name Flagyl, is used to treat the gastrointestinal infection and the activity against anaerobic bacteria like protozoa. The current work describes the interaction of metronidazole drug with water solvent needed for oral ingestion, which is the most common and convenient pathway for the administration of the drug in the body. Computational calculations are performed to optimize the metronidazole drug having solvent (water) at different positions. NBO and AIM calculations employed to determine the strength of intermolecular hydrogen bonding interactions between metronidazole and solvent (water). The second perturbation energy was calculated and the result was a maximum of 67 kJ mol-1 for O-H…O hydrogen bonding interaction. The solvation energy of the metronidazole drug, determined using the Solvation Model based on Density (SMD) model, is found to be -69.2 kJ mol-1. The bonding parameters of the solvated drug have been analysed through critical point calculations based on the Atom-in-Molecule (AIM) theory. Furthermore, an ab initio molecular dynamics (AIMD) study reveals that the lowest decomposition energies of metronidazole in the presence of water, considering all pores and different pores, are -927.86 and -699.003 a.u., respectively.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Department of Science and Technology, Ministry of Science and Technology, India
Grant numbers SRG/2019/002284
References
S. Löfmark, C. Edlund, C. E. Nord, Clin Infect Dis 50 (2010) S16 (http://dx.doi.org/10.1086/647939)
S. Arora, J. Clarke, E. Tsakalozou, P. Ghosh, K. Alam, J. E. Grice, M. S. Roberts, M. Jamei, S. Polak, Mol. Pharm. 19 (2022) 3139 (http://dx.doi.org/10.1021/acs.molpharmaceut.2c00229)
C. Gao, X. Wang, B. Yang, W. Yuan, W. Huang, G. Wu, J. Ma, ACS Nano 17 (2023) 7335 (http://dx.doi.org/10.1021/acsnano.2c11305)
D. Pal, S. Banerjee, J. Cui, A. Schwartz, S. K. Ghosh, J. Samuelson, Antimicrob. Agents Chemother. 53 (2009) 458 (http://dx.doi.org/10.1128/aac.00909-08)
J. Samuelson, Antimicrob. Agents Chemother. 43 (1999) 1533 (http://dx.doi.org/10.1128/aac.43.7.1533)
R. Nau, F. Sörgel, H. Eiffert, Clinical microbiology reviews 23 (2010) 858 (http://dx.doi.org/10.1128/cmr.00007-10)
S. V. Sastry, J. R. Nyshadham, J. A. Fix, Pharmaceutical Science & Technology Today 3 (2000) 138 (http://dx.doi.org/10.1016/S1461-5347(00)00247-9)
B. Agoram, W. S. Woltosz, M. B. Bolger, Adv. Drug Delivery Rev. 50 (2001) S41 (http://dx.doi.org/10.1016/S0169-409X(01)00179-X)
M. Jamei, D. Turner, J. Yang, S. Neuhoff, S. Polak, A. Rostami-Hodjegan, G. Tucker, The AAPS Journal 11 (2009) 225 (http://dx.doi.org/10.1208/s12248-009-9099-y)
C.-E. Chang, M. K. Gilson, J. Comput. Chem. 24 (2003) 1987 (http://dx.doi.org/10.1002/jcc.10325)
S. L. Mayo, B. D. Olafson, W. A. Goddard, J. Phys. Chem. 94 (1990) 8897 (http://dx.doi.org/10.1021/j100389a010)
Y. J. Franzke, C. Holzer, J. H. Andersen, T. Begušić, F. Bruder, S. Coriani, F. Della Sala, E. Fabiano, D. A. Fedotov, S. Fürst, J. Chem. Theory Comput. 19 (2023) 6859 (http://dx.doi.org/10.1021/acs.jctc.3c00347)
S. G. Balasubramani, G. P. Chen, S. Coriani, M. Diedenhofen, M. S. Frank, Y. J. Franzke, F. Furche, R. Grotjahn, M. E. Harding, C. Hättig, J. Chem. Phys. 152 (2020) 184107 (http://dx.doi.org/10.1063/5.0004635)
Gaussian 09, Revision A.02, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016.
C. Steffen, K. Thomas, U. Huniar, A. Hellweg, O. Rubner, A. Schroer, J. Comput. Chem. 31 (2010) 2967 (http://dx.doi.org/10.1002/jcc.21576)
GaussView, Roy Dennington, Todd A. Keith, and John M. Millam, Semichem Inc., Shawnee Mission, KS, 2016.
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, J. Phys. Chem. 98 (1994) 11623 (https://doi.org/10.1021/j100096a001)
Y. Zhao, N. E. Schultz, D. G. Truhlar, J. Chem. Theory Comput. 2 (2006) 364 (http://dx.doi.org/10.1021/ct0502763)
S. Grimme, J. Comput. Chem. 27 (2006) 1787 (https:/doi.org/10.1002/jcc.20495)
M. J. Frisch, M. Head-Gordon, J. A. Pople, Chem. Phys. Lett. 166 (1990) 281 (https:/doi.org/10.1016/0009-2614(90)80030-H)
M. J. Frisch, M. Head-Gordon, J. A. Pople, Chem. Phys. Lett. 166 (1990) 275 (https:/doi.org/10.1016/0009-2614(90)80029-D)
D. E. Woon, T. H. Dunning, Jr., J. Chem. Phys. 98 (1993) 1358 (http://dx.doi.org/10.1063/1.464303)
R. A. Kendall, T. H. Dunning, Jr., R. J. Harrison, J. Chem. Phys. 96 (1992) 6796 (http://dx.doi.org/10.1063/1.462569)
T. H. Dunning, Jr., J. Chem. Phys. 90 (1989) 1007 (http://dx.doi.org/10.1063/1.456153)
S. F. Boys, F. and Bernardi, Mol. Phys. 19 (1970) 553 (http://dx.doi.org/10.1080/00268977000101561)
F. Weinhold, J. Comput. Chem. 33 (2012) 2363 (http://dx.doi.org/10.1002/jcc.23060)
E. D. Glendening, C. R. Landis, F. Weinhold, J. Comput. Chem. 40 (2019) 2234 (http://dx.doi.org/10.1002/jcc.25873)
R. A. Boto, F. Peccati, R. Laplaza, C. Quan, A. Carbone, J. P. Piquemal, Y. Maday, A. J. Contreras-Garci, J. Chem. Theory Comput. 16 (2020) 4150 (http://dx.doi.org/10.1021/acs.jctc.0c00063)
F. Weinhold, C. Landis, E. Glendening, Int. Rev. Phys. Chem. 35 (2016) 399 (https://doi.org/10.1080/0144235X.2016.1192262)
P. Kolandaivel, V. Nirmala, J. Mol. Struct. 694 (2004) 33 (http://dx.doi.org/10.1016/j.molstruc.2004.01.030)
P. Su, H. Li, J. Chem. Phys. 131 (2009) 014102 (http://dx.doi.org/10.1063/1.3159673)
T. Lu, J. Chem. Phys. 161 (2024) (http://dx.doi.org/10.1063/5.0216272)
T. Lu, Q. Chen, Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory 2 (2022) 631
T. Lu, F. Chen, J. Comput. Chem. 33 (2012) 580 (http://dx.doi.org/10.1002/jcc.22885)
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, J. A. Montgomery Jr, J. Comput. Chem. 14 (1993) 1347 (https:/doi.org/10.1002/jcc.540141112)
V. Barone, M. Cossi, J. Phys. Chem. A 102 (1998) 1995 (http://dx.doi.org/10.1021/jp9716997)
A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. B 113 (2009) 6378 (http://dx.doi.org/10.1021/jp810292n)
F. Weigend, Phys. Chem. Chem. Phys. 8 (2006) 1057 (http://dx.doi.org/10.1039/B515623H)
S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 32 (2011) 1456 (https:/doi.org/10.1002/jcc.21759)
C. Adamo, V. Barone, J. Chem. Phys. 110 (1999) 6158 (http://dx.doi.org/10.1063/1.478522)
D. Marx, J. Hutter, Ab initio molecular dynamics: basic theory and advanced methods. Cambridge University Press: 2009. (http://dx.doi.org/10.1017/CBO9780511609633)
P. Carloni, U. Rothlisberger, M. Parrinello, Acc. Chem. Res. 35 (2002) 455 (http://dx.doi.org/10.1021/ar010018u)
F. Neese, Wiley Interdiscip. Rev. Comput. Mol. Sci. 12 (2022) e1606 (http://dx.doi.org/10.1002/wcms.1606)
S. Kumar, J. Serb. Chem. Soc. 88 (2022) 381 (http://dx.doi.org/10.2298/JSC220921087K)
A. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. (Washington, DC, U. S.) 88 (1988) 899 (http://dx.doi.org/10.1021/cr00088a005)
S. Kumar Panja, S. Kumar, A. D. Fazal, S. Bera, J. Photochem. Photobiol., A 445 (2023) 115084 (http://dx.doi.org/10.1016/j.jphotochem.2023.115084)
P. L. A. Popelier, F. Aicken, S. O’Brien, Atoms in molecules. Prentice Hall Manchester: 2000; Vol. 188.