Quantum-chemical study of C–H···O interactions between HTcO4 and aromatic amino acids Scientific paper
Main Article Content
Abstract
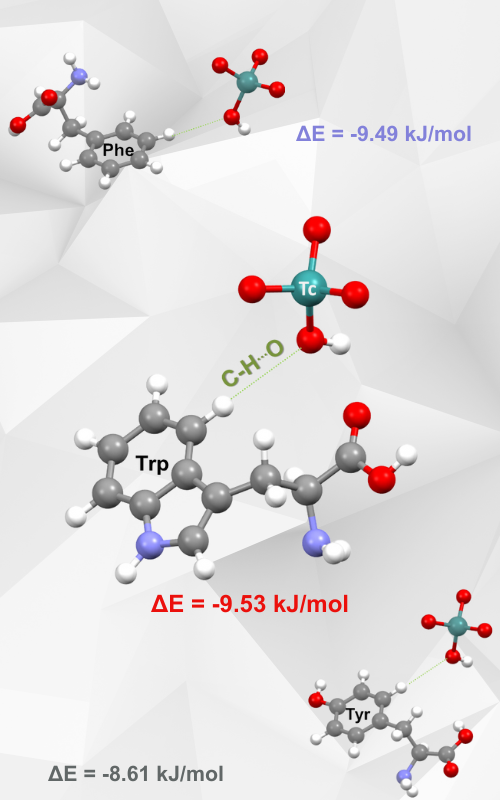
This study investigates C–H···O interactions between HTcO4 and aromatic amino acids (phenylalanine, tyrosine and tryptophan) through quantum-chemical calculations. The interaction energies calculations were combined with the analysis of molecular electrostatic potentials (MEP) to understand the nature of these interactions. The strongest interaction was observed for the HTcO4–tryptophan with an energy minimum of –9.53 kJ/mol at a distance of 2.1 Å. Phenylalanine showed a similarly strong interaction, with a minimum of –9.49 kJ/mol, while tyrosine exhibited the weakest interaction, with a minimum of –8.61 kJ/mol. Electrostatic potential maps confirmed the electrostatic nature of the C–H···O interactions, highlighting the role of the oxygen atoms in acting as hydrogen bond acceptors. These findings suggest that the position of the hydrogen atoms relative to the substituents on the aromatic ring influences the strength of the interactions. The results presented here could be of great importance for the recognition of new, overlooked noncovalent contacts between pertechnetic acid and amino acid fragments and a better understanding of the stability of pertechnetate-peptide complexes.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200026;451-03-66/2024-03/200168
References
D. Papagiannopoulou, J. Label. Compd. Radiopharm. 60 (2017) 502 (https://doi.org/10.1002/jlcr.3531).
J. G. O'Connell-Danes, B. T. Ngwenya, C. A. Morrison, J. B. Love, Angew. Chem. Int. Ed. 63 (2024) e202409834 (https://doi.org/10.1002/anie.202409834)
S. M. Rathmann, Z. Ahmad, S. Slikboer, H. A. Bilton, D. P. Snider, J. F. Valliant, in Radiopharmaceutical Chemistry, J. Lewis, A. Windhorst, B. Zeglis, Eds., Springer, Cham., 2019, p. 311 (https://doi.org/10.1007/978-3-319-98947-1_18)
J. Chen, Z. Cheng, Y. Miao, S. S. Jurisson, T. P. Quinn, Cancer 94 (2002) 1196 (https://doi.org/10.1002/cncr.10284)
D. Grödler, S. Burguera, A. Frontera, E. Strub, Chem. Eur. J. 30 (2024) e202400100 (https://doi.org/10.1002/chem.202400100)
R. Waibel, R. Alberto, J. Willuda, R. Finnern, R. Schibli, A. Stichelberger, A. Egli, U. Abram, J.-P. Mach, A. Plückthun, P. A. Schubiger, Nat. Biotechnol. 17 (1999) 897 (https://doi.org/10.1038/12890)
M. Bartholomä, J. Valliant, K. P. Maresca, J. Babich, J. Zubieta, Chem. Commun. 5 (2009) 493 (https://doi.org/10.1039/B814903H)
M. B.-U. Surfraz, R. King, S. J. Mather, S. Biagini, P. J. Blower, J. Inorg. Biochem. 103 (2009) 971 (https://doi.org/10.1016/j.jinorgbio.2009.04.007)
D. Hernández-Valdés, R. Alberto, U. Jáuregui-Haza, RSC Adv. 6 (2016) 107127 (https://doi.org/10.1039/C6RA23142J)
K. Manikandan, S. Ramakumar, Proteins 56 (2004) 768 (https://doi.org/10.1002/prot.20152)
D. Ž. Veljković, G. V. Janjić, S. D. Zarić, CrystEngComm 13 (2011) 5005 (https://doi.org/10.1039/C1CE05065F)
D. Ž. Veljković, J. Mol. Graph. Model. 80 (2018) 121 (https://doi.org/10.1016/j.jmgm.2017.12.014)
D. Ž. Veljković, V. B. Medaković, J. M. Andrić, S. D. Zarić, CrystEngComm 16 (2014) 10089 (https://doi.org/10.1039/C4CE00595C)
J. Lj. Dragelj, I. M. Stanković, D. M. Božinovski, T. Meyer, D. Ž. Veljković, V. B. Medaković, E.-W. Knapp, Snežana D. Zarić, Cryst. Growth Des. 16 (2016) 1948 (https://doi.org/10.1021/acs.cgd.5b01543)
Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013
C. Moller, M. S. Plesset, Phys. Rev. 46 (1934) 618 (https://doi.org/10.1103/PhysRev.46.618)
F. Weigend, Phys. Chem. Chem. Phys. 8 (2006) 1057 (https://doi.org/10.1039/B515623H)
T. H. Dunning Jr., J. Chem. Phys. 90 (1989) 1007 (https://doi.org/10.1063/1.456153)
S. F. Boys, F. Bernardi, Mol. Phys. 19 (1970) 553 (https://doi.org/10.1080/00268977000101561)
L. Laaksonen, J. Mol. Graphics 10 (1992) 33 (https://doi.org/10.1016/0263-7855(92)80007-Z)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Cryst., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954).