Binding elucidation of azo dye with DNA via spectroscopic approaches and molecular docking techniques Scientific paper
Main Article Content
Abstract
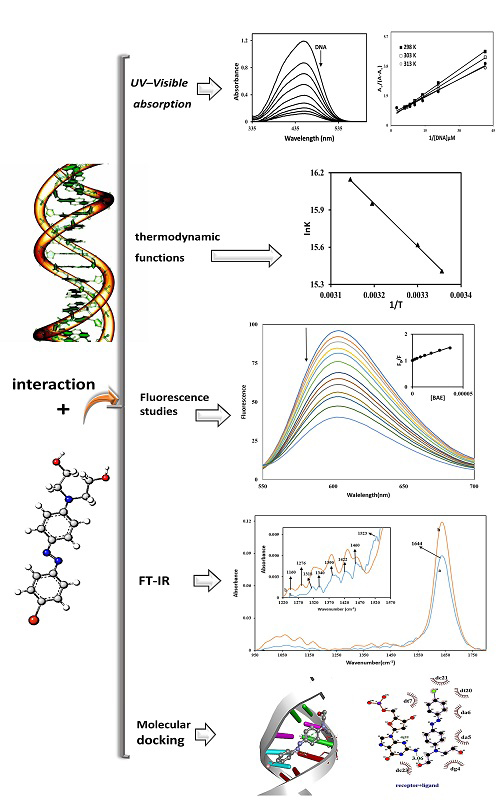
In this study, the interaction between a synthesized monoazo disperse dye and calf thymus DNA (Ct-DNA) was investigated via UV‒Vis spectroscopy, fluorescence and FT-IR spectroscopy and molecular docking calculations. Hypochromic effects on the absorbance and quenching of fluorescence were observed, revealing the binding of azo dye to Ct-DNA. Upon the addition of Ct-DNA, the azo dye showed a hypochromic effect and a small redshift in the wavelength of the absorption spectra, suggesting a groove binding mode of interaction of this probe with Ct-DNA, which was confirmed by the molecular docking results. The values of the binding constant were calculated from the maximum absorption spectra of the azo dye at various Ct-DNA concentrations at several temperatures and the corresponding thermodynamic parameters ΔG°, ΔH° and ΔS° were obtained. In addition, fluorescence resonance energy transfer showed that the distance between the donor (EB–Ct-DNA) and acceptor (azo dye) is suitable for energy transfer. Molecular docking analysis revealed that hydrogen bonds and π-electrons on the benzene ring of the azo dye are crucial for binding the azo dye to Ct-DNA. The results of the molecular docking investigations corroborate well with those of the spectral studies and these probes bind to the minor groove of Ct-DNA.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
A. Travers , G. Muskhelishvili, FEBS J. 282 (2015) 2279 (https://doi.org/10.1111/febs.13307)
C. Nieuwland, T. A. Hamlin , C. F. Guerra , G. Barone , F. M. Bickelhaupt, Chem. Open 11 (2022) 2 (https://doi.org/10.1002/open.202100231)
S. Minchin, J. Lodge, Essays Biochem. 63 (2019) 433 (https://doi.org/10.1042/EBC20180038)
S. Gupta, S. Aggarwal, M. Munde, ACS Omega 8 (2023) 4554 (http://doi.org/10.1021/acsomega.2c01557)
K. Shridhar, G. K.Walia, A. Aggarwal, S. Gulati, A. Geetha, D. Prabhakaran, P. K. Dhillon, P. Rajaraman, Oral Oncology 53 (2016) 1 (http://doi.org/10.1016/j.oraloncology.2015.11.012)
S. Benkhaya, S. E. Mrabet, A. Harfi, Heliyon 6 (2020) 03271 (http://doi.org/10.1016/j.heliyon.2020.e03271)
Y. X. Liu, H. W. Mo, Z. Lv, F. Shen, C. L. Zhan, Y. Y. Qi, Z. Mao, X. Y. Le, Trans. Met. Chem. 43 (2018) 259 (http://doi.org/10.1007/s11243-018-0211-y)
M. Maliyappa, J. Keshavayya, N. Mallikarjuna, P. M. Krishna, N. Shivakumara, T. Sandeep, K. Sailaja, M. A. Nazrulla, J. Mol Struct. 1179 (2019) 630 (http://doi.org/10.1016/j.molstruc.2018.11.041)
A. S. Mondal, A. K. Pramanik, L. Patra, C. K. Manna, T. K. Mondal, J. Mol. Struct. 1146 (2017) 146 (http://doi.org/10.1016/j.molstruc.2017.05.131)
Y. Ali, S. Abd Hamid, U. Rashid, Med. Chem. 18 (2018) 1548 (http://doi.org/10.2174/1389557518666180524113111)
M. Y. Zhao, Y. F. Tang, G. Z. Han, Molecules 28 (2023) 6741 (http://doi.org/10.3390/molecules28186741)
N. Venugopal, G. Krishnamurthy, H. S. Bhojya Naik, J. D. Manohara, J. Inorg. Organomet. Polym. Mat. 30 (2020) 2608 (http://doi.org/10.1007/s10904-019-01394-8)
S. Qamar, Z. Akhter, S. Yousuf, H. Bano, F. Perveen, J. Mol. Struct. 1197 (2019) 345 (https://doi.org/10.1016/j.molstruc.2019.07.069)
S. Benkhaya, S. M'rabet, A. El Harfi. Heliyon 6 (2020) 3072. (https://doi.org/10.1016/j.heliyon.2020.e03271)
S. Das, S. Chatterjee, S. Pramanik, P. S. Devi, G. S. Kumar, J. Photochem. Photobiol., B 178 (2018) 339 (https://doi.org/10.1016/j.jphotobiol.2017.10.039)
M. R. Yazdanbakhsh, A. Mohammadi, J. Mol. Liq. 148 (2009) 35 (http://doi.org/10.1016/j.molliq.2009.06.001)
J. Albani, Principles and applications of fluorescence spectroscopy, Wiley-Blackwell, Hoboken, NJ, 2007 (http://doi.org/10.1002/9780470692059)
B. Kavitha, M. Sravanthi, P. Saritha Redd, J. Mol. Struct. 1185 (2019) 153 (https://doi.org/10.1016/j.molstruc.2019.02.093)
L. Hana, Y. Zhoub, X. Huangb, M. Xiaoa, L. Zhoua, J. Zhoua, A. Wangc, J. Shena, Spectrochim. Acta, A 123 (2014) 497 (http://dx.doi.org/10.1016/j.saa.2013.11.088)
X. Li, Y. Yuan, Y. Wang, F. Zhang, R. Zhao, D. Shao, S. Bi, Process Biochem. 108 (2021) 26 (http://doi.org/10.1016/j.procbio.2021.05.023)
J. Antosiewicz, D. Shugar, Biophys. Rev. 8 (2016) 163 (http://doi.org/10.1007/s12551-016-0197-7)
Y. Pin, Z. Chunqiong, Acta Chim. Sin. 61 (2003) 1455 (in Chinese) (https://sioc-journal.cn/Jwk_hxxb/EN/Y2003/V61/I9/1455#1)
Z. Shokohi-Pour, H. Chiniforoshan, M. R. Sabzalian, S. A. Esmaeili, A. A. Momtazi-
-Borojeni, J. Biomol. Struct. Dyn. 36 (2018), 532 (http://dx.doi.org/10.1080/07391102.2017.1287006)
K. Xia, G. Zhang, S. Li, D. Gong, J. Fluoresc. 27 (2017) 1815 (http://doi.org/10.1007/s10895-017-2119-x)
P. D. Ross, S. Subramanian, Biochemistry 20 (1981) 3096 (http://doi.org/10.1021/bi00514a017)
B. Shen, H. Yang, J. C. X. Liu, M. Zhou, Spectrochim. Acta, A 261 (2021) 1386 (https://doi.org/10.1016/j.saa.2021.119998)
S. Ansari, I. Yousuf, F. Arjmand, M. K. Siddiqi, S. Naqvi, Int. J. Biol. Macromol. 116 (2018) 1105 (http://doi.org/10.1016/j.ijbiomac.2018.05.052)
W. R. Ware, J. Phys. Chem. 66 (1962) 455 (https://doi.org/10.1021/j100809a020)
W. He, Y. Li, J. Tang, F. Luan, J. Jin, Z. Hu, Int. J. Biol. Macromol. 39 (2006) 165 (https://doi.org/10.1016/j.ijbiomac.2005.11.003)
X. Y. Cao, S. Wang, S. Q. Tian, H. Lou, Y. C. Kong, Z. J. Yang, J. L. Liu, Spectrochim. Acta, A 203 (2018) 301 (http://doi.org/10.1016/j.saa.2018.05.091)
S. S. Ansari, I. Yousuf, F. Arjmand, M. K. Siddiqi, S. Naqvi, Int. J. Biol. Macromol. 116 (2018) 1105 (http://doi.org/10.1016/j.ijbiomac.2018.05.052)
H. Li, H. Dou, Y. Zhang, Z. Li, R. Wang, J. Chang, Spectrochim. Acta, A 136 (2015) 416 (https://doi.org/10.1016/j.saa.2014.09.051)
N. Vandenberk, A. Barth, D. Borrenberghs, J. Hofkens, J. Hendrix, J. Phys. Chem., B 122 (2018) 4249 (http://doi.org/10.1021/acs.jpcb.8b00108)
X. Li, Y. Yuan, Y. Wang, F. Zhang, R. Zhao, D. Shao, S. Bi, Process Biochem. 108 (2021) 26–33 (https://doi.org/10.1016/j.procbio.2021.05.023)
Principles of Fluorescence Spectroscopy, 3rd ed., J. R. Lakowicz, Ed., Springer, Boston, MA, 2006 (http://doi.org/10.1007/978-0-387-46312-4)
S. Bi, X. Li, Z. Ren, B. Yang, Y. Wang, F. Zhang, Y. Yuan, D. Shao, R. Zhao, Luminescence 37 (2022) 1275 (http://doi.org/10.1002/bio.4293)
E. Tymchenko, V. Glova, A. Soldatova, E. Chikhirzhina, A. Polyanichko, J. Phys.: Conf. Ser. 1400 (2019) 1742 (http://doi.org/10.1088/1742-6596/1400/3/033004)
S. Ponkarpagam, K. N. Vennila, K. P. Elango, Spectrochim. Acta, A 278 (2022) 121351 (http://doi.org/10.1016/j.saa.2022.121351)
B. Shen, H. Yang, J. Chen, X. Liu, M. Zhou, Spectrochim. Acta, A 261 (2021) 119998 (https://doi.org/10.1016/j.saa.2021.119998)
E. S. Istifli, Colorants 1 (2022) 236 (https://doi.org/10.3390/colorants1020015).