Computational insights into the inhibitory potential of dihydroorotate dehydrogenase by natural compounds in Artocarpus champeden as antimalarial agents Scientific paper
Main Article Content
Abstract
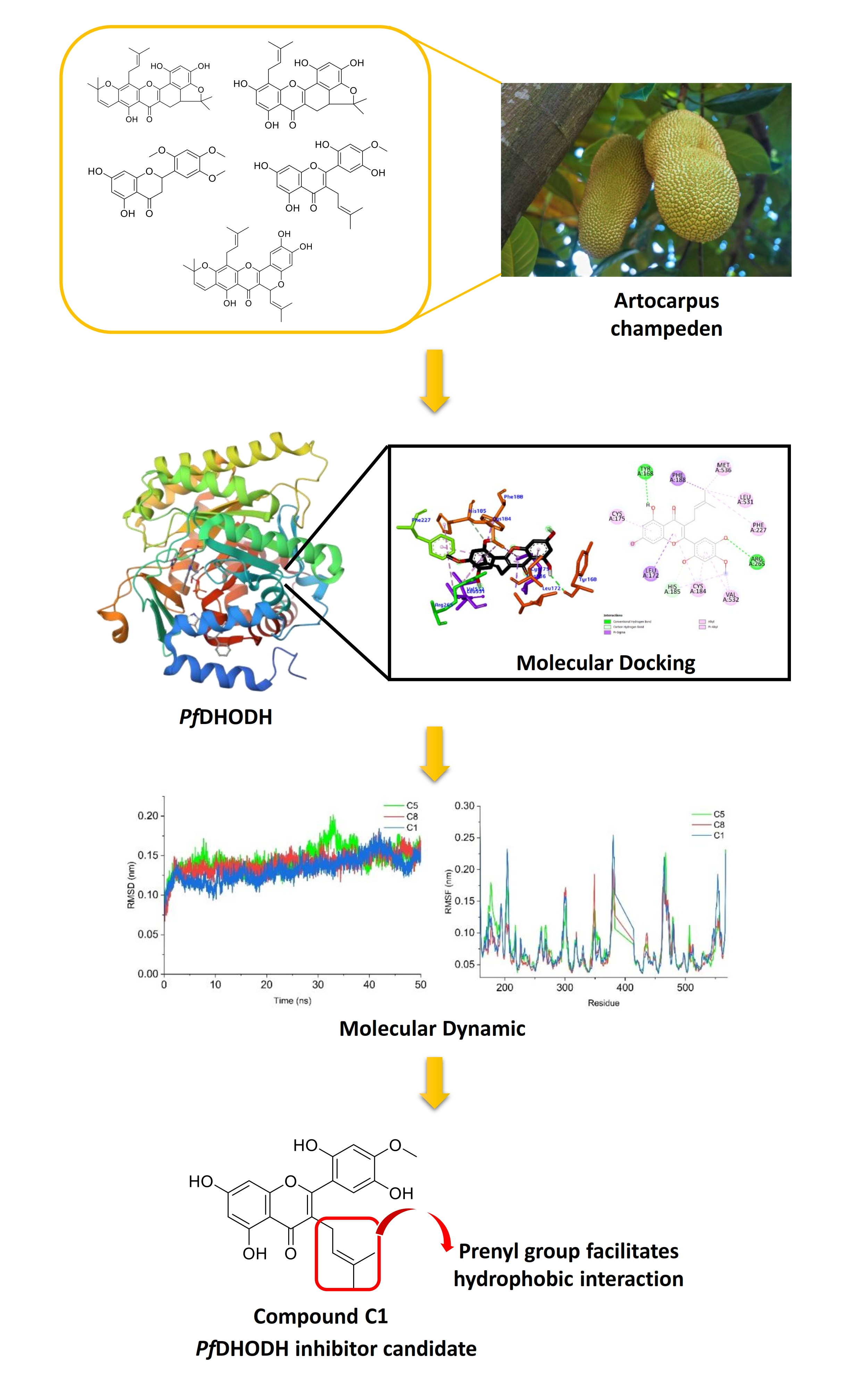
Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) is a crucial target for the development of antimalarial drugs, as it plays a significant role in inhibiting the growth of parasites by disrupting the production of pyrimidines in the bloodstream. Artocarpus champeden is known to contain prenylated flavonoids with potential antimalarial activity. This study aims to explore the chemical interactions of active compounds found in A. champeden through an in silico approach. Nine compounds were docked into PfDHODH (PDB ID: 6I55), and their stability was subsequently assessed using molecular dynamics simulations. Molecular docking results indicated that compounds C1, C5 and C6 emerged as the most promising candidates, exhibiting binding affinities of –37.80, –35.28 and –34.44 kJ/mol, respectively. His185 and Arg265 were found to be key binding residues, interacting with these compounds in a manner similar to DZB, the control ligand. A 50-ns molecular dynamics simulation further confirmed the stability of these compounds throughout the simulation. Moreover, the examination of hydrogen bond occupancy demonstrated that compound C1 consistently engaged in hydrogen bonding interactions with His185 and Arg265 throughout the simulation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
Т. Abdul-Rahman, O. A. Ajetunmobi, G. B. Bamigbade, I. Ayesiga, M. H. Shah, T. S. Rumide, A. B. Adesina, G. A. Adeshina, O. E. Oni, B. I. N. Christian, A. T. Aborode, A. A. Wireko, H. I. Thaalibi, I. M. Abdalla, S. B. Banimusa, J. N. Jonathan, I. A. Onifade, M. A. Haque, Int. J. Equity Health 24 (2025) 22 (https://doi.org/10.1186/s12939-025-02378-6)
WHO, World malaria report 2024, 2024
Kasus Malaria di Indonesia, Kementerian Kesehatan RI, https://malaria.kemkes.go.id/case (accessed: March 5, 2025)
A. R. Parhizgar, A. Tahghighi, Iran. J. Med. Sci. 42 (2017) 115 (https://pmc.ncbi.nlm.nih.gov/articles/PMC5366359/)
K. Pal, M. K. Raza, J. Legac, M. A. Rahman, S. Manzoor, P. J. Rosenthal, N. Hoda, RSC Med. Chem. 12 (2021) 970 (https://doi.org/10.1039/D1MD00038A)
M. Thellier, A. A. J. Gemegah, I. Tantaoui, J. Clin. Med. 13 (2024) 1 (https://doi.org/10.3390/JCM13195680)
R. W. van der Pluijm, C. Amaratunga, M. Dhorda, A. M. Dondorp, Trends Parasitol. 37 (2021) 15 (https://doi.org/10.1016/j.pt.2020.09.011)
M. Ouji, J. M. Augereau, L. Paloque, F. Benoit-Vical, Parasite 25 (2018) 1 (https://doi.org/10.1051/PARASITE/2018021)
Fitrya, A. Amriani, R. P. Novita, R. Gabriella, S. V. Lestari, A. Agustina, J. Ayurveda Integr. Med. 14 (2023) 1 (https://doi.org/10.1016/J.JAIM.2023.100746)
Q. F. Hu, S. Yao, Y. Y. Ma, R. F. Xiong, G. H. Kong, Y. P. Wu, G. K. Zhao, M. Dong, W. G. Wang, M. Zhou, Y. K. Li, Chem. Biol. Technol. Agric. 10 (2023) 94 (https://doi.org/10.1186/s40538-023-00457-w)
S. Supandi, M. S. Wulandari, E. Samsul, A. Azminah, R. Y. Purwoko, H. Herman, H. Kuncoro, A. Ibrahim, N. S. S. Ambarwati, R. Rosmalena, R. N. Azizah, S. Paramita, I. Ahmad, J. Adv. Pharm. Technol. Res. 13 (2022) 207 (https://doi.org/10.4103/JAPTR.JAPTR_376_22)
A. Widyawaruyanti, Subehan, S. K. Kalauni, S. Awale, M. Nindatu, N. C. Zaini, D. Syafruddin, P. B. S. Asih, Y. Tezuka, S. Kadota, J. Nat. Med. 61 (2007) 410 (https://doi.org/10.1007/s11418-007-0153-8)
M. M. Taek, MoluccaMedica 4 (2011) 37 (https://ejournal.unpatti.ac.id/ppr_iteminfo_lnk.php?id=571) in Indonesian
T. S. Wahyuni, W. Ekasari, A. Widyawaruyanti, Y. Hirasawa, H. Morita, N. C. Zaini, Heterocycles 79 (2009) 1121 (https://doi.org/10.3987/COM-08-S(D)72)
S. R. Krungkrai, J. Krungkrai, Asian Pac. J. Trop. Med. 9 (2016) 525 (https://doi.org/10.1016/J.APJTM.2016.04.012)
L. V. Hoelz, F. A. Calil, M. C. Nonato, L. C. Pinheiro, N. Boechat, Future Med. Chem. 10 (2018) 1853 (https://doi.org/10.4155/fmc-2017-0250)
C. D. Goodman, J. E. Siregar, V. Mollard, J. Vega-Rodríguez, D. Syafruddin, H. Matsuoka, M. Matsuzaki, T. Toyama, A. Sturm, A. Cozijnsen, M. Jacobs-Lorena, K. Kita, S. Marzuki, G. I. McFadden, Science 352 (2016) 349 (https://doi.org/10.1126/science.aad9279)
A. Blanshard, P. Hine, Cochrane Database Syst. Rev. 1 (2021) 1465 (https://doi.org/10.1002/14651858.cd004529.pub3)
P. H. M. Torres, A. C. R. Sodero, P. Jofily, F. P. Silva-Jr, Int. J. Mol. Sci. 20 (2019) 4574 (https://doi.org/10.3390/ijms20184574)
D. Van Der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark, H. J. C. Berendsen, J. Comput. Chem. 26 (2005) 1701 (https://doi.org/10.1002/jcc.20291)
V. Zoete, M. A. Cuendet, A. Grosdidier, O. Michielin, J. Comput. Chem. 32 (2011) 2359 (https://doi.org/10.1002/JCC.21816)
K. Vanommeslaeghe, E. Hatcher, C. Acharya, S. Kundu, S. Zhong, J. Shim, E. Darian, O. Guvench, P. Lopes, I. Vorobyov, A. D. Mackerell, J. Comput. Chem. 31 (2010) 671 (https://doi.org/10.1002/JCC.21367)
S. Boonstra, P. R. Onck, E. Van Der Giessen, J. Phys. Chem., B 120 (2016) 3692 (https://doi.org/10.1021/acs.jpcb.6b01316)
P. J. P. Tjitda, F. O. Nitbani, T. D. Wahyuningsih, R. I. Lerrick, Y. M. Abanit, ChemistrySelect 10 (2025) 1 (https://doi.org/10.1002/slct.202404851)
P. J. P. Tjitda, F. O. Nıtbanı, A. A. Parıkesıt, M. I. T. Bessı, T. D. Wahyunıngsıh, Trop. J. Nat. Prod. Res. 8 (2024) 6208 (https://doi.org/10.26538/TJNPR/V8I2.18)
L. P. Hastuti, F. Hermawan, M. R. Iresha, T. Ernawati, Firdayani, Informatics Med. Unlocked 47 (2024) 1 (https://doi.org/10.1016/J.IMU.2024.101485)
R. L. S. Shrestha, P. Neupane, S. Dhital, N. Parajuli, B. Maharjan, T. Shrestha, S. Bharati, B. P. Marasini, J. A. Subin, Moroccan J. Chem. 12 (2024) 1742 (https://doi.org/10.48317/IMIST.PRSM/MORJCHEM-V12I4.48008)
V. K. Vyas, T. Shukla, K. Tulsian, M. Sharma, S. Patel, Comput. Biol. Chem. 101 (2022) 107787 (https://doi.org/10.1016/J.COMPBIOLCHEM.2022.107787).