The use of mucilage extracted from Opuntia ficus indica as microencapsulating shell
Main Article Content
Abstract
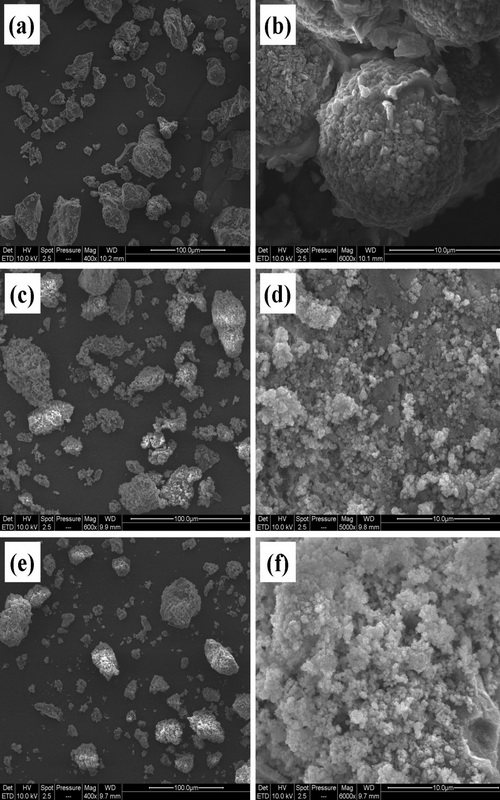
This study was aimed at investigating the micro-formulation of capsules using natural biopolymers, such as cactus mucilage (CM), carboxymethyl cellulose sodium salt (CMCNa) and chitosan (Chi) as the wall material, for the transport and supply of sunflower oil. CM samples were extracted from Opuntia ficus indica (OFI) by precipitation at different supernatant pH values (2, 4 and 12). The extracted natural polysaccharide and the resulting microcapsules were characterized by different experimental techniques. Fourier transform infrared spectroscopy analysis of the CM showed the presence of uronic acid units and sugars. Scanning electron microscopy revealed that most particles were adhered together, causing the formation of compact, linked agglomerates, which resulted in different microstructures with irregular shapes. All oil–core microcapsules were characterized, and the results showed that the different shell materials could be used to microencapsulate sunflower oil. Among them, the microcapsule crosslinked with CM and Chi was the most suitable, with the highest encapsulation efficiency (95 %). This coacervation led to the narrowest size distribution of the capsules, with diameters ranging from 1 to 5 μm. Optical microscopy confirmed the deposition of coacervate droplets around oil drops and clearly showed that the formation of coacervated particles and their deposition onto oil droplets were successive events.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
C. Thies, in Encyclopiedia of Polymer Science Engineering, H. F. Mark, N. M. Bikales, C. G. Overberger, G. Menges (Eds.), 2nd ed., Vol. 9, Wiley, 1987. pp. 724–745 (ISBN: 9780471809418)
W. C. Griffin, Solid essential oil concentrate and process of preparing the same (1951), U. S. Patent No. 2,556,410
B. K. Green, L. Scheleicher, Manifold record material (1956), U. S. Patent No. 2,730,456
A. Gharsallaoui, Food Res. Int. 40 (2007) 1107 (https://doi. org/10. 1016/j. foodres. 2007. 07. 004)
R. R. Esfahani, H. Jun, S. Rahmani, A. Miller, J. Lahann, ACS Omega 30 (2017) 2839 (https://doi. org/10. 1021/acsomega. 7b00570)
B. Gomez, F. J. Barba, R. Domínguez, P. Putnik, D. B. Kovacevic, M. Pateiro, F. Toldra, J. M. Lorenzo, Trends Food Sci. Tech. 82 (2018) 135 (https://doi. org/10. 1016/j. tifs. 2018. 10. 006)
G. Ozkan, P. Franco, I. De Marco, J. Xiao, E. Capanoglu, Food Chem. 272 (2019) 494 (https://doi. org/10. 1016/j. foodchem. 2018. 07. 205)
G. Orive, E. Santos, J. L. Pedraz, R. M. Hernandez, Adv. Drug Deliver Rev. 67–68 (2014) 3 (https://doi. org/10. 1016/j. addr. 2013. 07. 009)
L. Zhao, J. Luo, H. Wang, G. Song, G. Tang, Appl. Therm. Eng. 99 (2016) 495 (http://dx. doi. org/10. 1016%2Fj. applthermaleng. 2015. 12. 111)
A. Nesterenko, I. Alric, F. Silvestre, V. Durrieu, Ind. Crop. Prod. 42 (2013) 469 (https://doi. org/10. 1016/j. indcrop. 2012. 06. 035)
B. N. Estevinho, F. Rocha, Application of Biopolymers in Microencapsulation Processes, Biopolymers for Food Design, Handbook of Food Bioengineering, Elsevier, 2018, p. 191 (ISBN: 9780128115015)
T. A. Comunian, C. S. Favaro-Trindade, Food Hydrocolloids 61 (2016) 442 (https://doi. org/10. 1016/j. foodhyd. 2016. 06. 003)
I. J. Joye, D. J. McClements, Curr. Opin. Colloid Interface Sci. 19 (2014) 417 (https://doi. org/10. 1016/j. cocis. 2014. 07. 002)
G. K. Kouassi, V. Gogineni, T. Ahmad, N. M. Gowda, M. S. Boley, N. Koissi, in Advances in Applied Nanotechnology for Agriculture; Б. Park, М. Appell (Eds.), ACS Symposium Series 1143 (2013) pp. 221–234
C. Schmitt, C. Sanchez, S. Desobry-Banon, J. Hardy, Crit. Rev. Food Sci. 38 (1998) 689 (https://doi. org/10. 1080/10408699891274354)
R. Khiari, N. Meksi, M. F. Mhenni, M. N. Belgacem, E. Mauret, Fiber Polym. 12 (2011) 587 (https://doi. org/10. 1007/s12221-011-0587-1)
S. Mansouri, R. Khiari, F. Bettaieb, A. El-Gendy, M. F. Mhenni, J. Polym. Environ. 23 (2015) 190 (https://doi. org/10. 1007/s10924-014-0691-6)
I. Moussa, R. Khiari, A. Moussa, M. N. Belgacem, M. F. Mhenni, Fiber. Polym. 20 (2019) 933 (https://doi. org/10. 1007/s12221-019-8665-x)
L. Medina-Torres, E. Brito-De La Fuente, B. Torrestiana-Sanchez, R. Katthain, Food Hydrocolloids 14 (2000) 417 (https://doi. org/10. 1016/S0268-005X(00)00015-1)
C. Sáenz, E. Sepúlveda, B. Matsuhiro, J. Arid Environ. 57 (2004) 275 (https://doi. org/10. 1016/S0140-1963(03)00106-X)
I. Sanchez-Ortega, B. E. Garcia-Almendarez, E. M. Santos-Lopez, L. R. Reyes-Gonzalez, C. Regalado, Food Hydrocolloids 52 (2016) 906 (https://doi. org/10. 1016/j. foodhyd. 2015. 09. 004)
A. Bernardino-Nicanor, E. N. Hinojosa-Hernandez, J. M. S. Juarez-Goiz, J. L. Montanez-Soto, M. E. Ramirez-Ortiz, L. Gonzalez-Cruz, J. Food Sci. Tech. 52 (2015) 343 (https://doi. org/10. 1007/s13197-013-0989-8)
A. K. Nayak, D. Pal, D. R. Pany, B. Mohanty, J. Adv. Pharm. Technol. Res. 1 (2010) 338 (https://dx. doi. org/10. 4103%2F0110-5558. 72430)
F. Mannai, M. Ammar, J. G. Yanez, E. Elaloui, Y. Moussaoui, J. Polym. Environ. 26 (2018) 798 (https://doi. org/10. 1007/s10924-017-0968-7)
E. K. Bae, S. J. Lee, J. Microencapsul. 25 (2008) 549 (https://doi. org/10. 1080/02652040802075682)
H. C. F. Carneiro, R. V. Tonon, C. R. F. Grosso, M. D. Hubinger, J. Food Eng. 115 (2013) 443 (https://doi. org/10. 1016/j. jfoodeng. 2012. 03. 033)
N. Bayar, M. Kriaa, R. Kammoun, Int. J. Biol. Macromol. 92 (2016) 441 (https://doi. org/10. 1016/j. ijbiomac. 2016. 07. 042)
N. Bayar, T. Bouallegue, M. Achour, M. Kriaa, R. Kammoun, A. Bougatef, Food Chem. 235 (2017) 275 (https://doi. org/10. 1016/j. foodchem. 2017. 05. 029)
O. Ishurd, F. Zgheel, M. Elghazoun, M. Elmabruk, A. Kermagi, J. F. Kennedy, C. J. Knill, Carbohyd. Polym. 82 (2010) 848 (https://doi. org/10. 1016/j. carbpol. 2010. 06. 006)
H. Zeng, S. Miao, Y. Zhang, S. Lin, Y. Jian, Y. Tian, B. Zheng, Food Hydrocolloids 52 (2016) 126 (https://doi. org/10. 1016/j. foodhyd. 2015. 05. 028)
M. M. Zhao, N. Yang, B. Yang, Y. Jiang, G. Zhang, Food Chem. 105 (2007) 1480 (https://doi. org/10. 1016/j. foodchem. 2007. 05. 031)
J. L. Rivera-Corona, F. Rodríguez-Gonzalez, R. Rendon-Villalobos, E. García-Her-nan¬dez, J. Solorza-Feria, LWT - Food Sci. Technol. 59 (2014) 806 (https://doi. org/10. 1016/j. lwt. 2014. 06. 011)
M. C. Otálora, J. A. G. Castaño, A. Wilches-Torres, LWT - Food Sci. Technol. 112 (2019) 108234 (https://doi. org/10. 1016/j. lwt. 2019. 06. 001)
V. E. Manhivi, S. Venter, E. O. Amonsou, T. Kudanga, Carbohyd. Polym. 195 (2018) 163 (https://doi. org/10. 1016/j. carbpol. 2018. 04. 062)
R. Gheribi, L. Puchot, P. Verge, N. Jaoued-Grayaa, M. Mezni, Y. Habibi, K. Khwaldia, Carbohyd. Polym. 190 (2018) 204 (https://doi. org/10. 1016/j. carbpol. 2018. 02. 085)
Q. Guo, S. W. Cui, Q. Wang, X. Hu, Q. Guo, K. Ji, R. Yada, Carbohyd. Polym. 86 (2011) 831 (https://doi. org/10. 1016/j. carbpol. 2011. 05. 034)
Y. L. Han, J. Gao, Y. Y. Yin, Z. Y. Jin, X. M. Xu, H. Q. Chen, Carbohyd. Polym. 151 (2016) 381 (https://doi. org/10. 1016/j. carbpol. 2016. 05. 085)
F. Mannai, M. Ammar, J. G. Yanez, E. Elaloui, Y. Moussaoui, Cellulose 23 (2016) 2061 (https://doi. org/10. 1007/s10570-016-0899-9)
A. Du Toit, M. De Wit, A. Hugo, Molecules 23 (2018) 916 (https://doi. org/10. 3390/molecules23040916)
J. Y. Yin, S. P. Nie, J. Li, C. Li, S. W. Cui, M. Y. Xie, J. Agr. Food Chem. 60 (2012) 7981 (https://doi. org/10. 1021/jf302052t)
H. Guo, X. Zhao, J. Microencapsul. 25 (2008) 221m (https://doi. org/10. 1080/02652040701861828)
J. C. Roy, F. Salaün, S. Giraud, A. Ferri, J. Guan, Carbohyd. Polym. 173 (2017) 202 (https://doi. org/10. 1016/j. carbpol. 2017. 06. 001)
H. Zhang, X. Wang, Sol. Energy Mat. Sol. Cells 93 (2009) 1366 (https://doi. org/10. 1016/j. solmat. 2009. 02. 021).