Urea as a complexing agent for selective removal of Ta and Cu in sodium carbonate based alumina chemical–mechanical planarization slurry Scientific paper
Main Article Content
Abstract
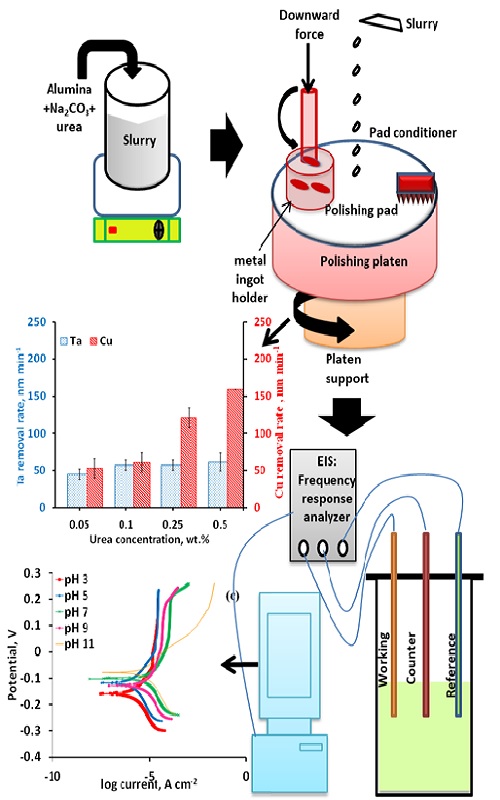
This work reports urea as a promising complexing agent in sodium carbonate-based alumina slurry for chemical–mechanical planarization (CMP) of tantalum and copper. Ta and Cu were polished using Na2CO3 (1 wt. %) with alumina (2 wt. %) in the presence and absence of urea. The effect of slurry pH, urea concentration, applied downward pressure and platen rotational speed were deliberated and the outcomes conveyed. Prior to the addition of urea, the Ta removal rate (RR) was observed to increase with pH from acidic to alkaline, having a maximum RR at pH 11. However, Cu RR decreases with increasing pH with minimum RR at pH 11. With the addition of urea in the slurry, a Cu to Ta removal rate selectivity of nearly 1:1 was encountered at pH 11. The addition of urea simultaneous boosts the Ta RR and suppresses Cu RR at pH 11, as it adsorbs on the metal surface. Potentiodynamic polarization was conducted to determine the corrosion current (Icorr) and the corrosion potential (Ecorr). Electrochemical impedance spectroscopy of both metals was carried out in the proposed formulation and the obtained outcomes are elaborated.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. J. Kim, Y. J. Jang, J. Choi, B. Kwon, K. Lee, Y. Ko, Met. Mater. Int. 335 (2013) 19 (https://doi.org/10.1016/j.jiec.2016.06.011)
M. Krishnan, J. W. Nalaskowski, L. M. Cook, Chem. Rev. 178 (2010) 110 (https://doi.org/10.1021/cr900170)
J. Seo, U. Paik, in Advances in Chemical Mechanical Planarization (CMP), S. Babu, Ed., Woodhead Publishing, Singapore, 2016, pp. 273–298 (https://doi.org/10.1016/B978-0-08-100165-3.00011-5)
J. M. Steigerwald, S. P. Murarka, R. J. Gutmann, Chemical Mechanical Planarization of Microelectronic Materials, John Wiley and Sons, Berlin, 2008 (ISBN-13: 978-00471-13827-3)
S. G. Pyo, Met. Mater. Int. 293 (2010) 2 (https://doi.org/10.1007/s12540-010-0420-1)
K. Maex, M. R. Baklanov, D. Shamiryan, F. Lacopi, S. H. Brongersma, Z. S. Yanovitskaya, J. Appl. Phys. 8793 (2003) 11 (https://doi.org/10.1063/1.1567460)
J. Cheng, T. Wang, L. Jiang, X. Lu, Appl. Sur. Sci. 401 (2015) 351 (https://doi.org/10.1016/j.apsusc.2015.05.150)
A. Vijayakumar, T. Du, K. B. Sundaram, Microelectron. Eng. 93 (2003) 70 (https://doi.org/10.1016/S0167-9317(03)00398-8)
R. Govindarajan, S. Siddiqui, M. Keswani, S. Raghavan, D. R. P. Singh, N. Chawla, Electrochem. Solid-State Lett. 10 (2011) 14 (https://doi.org/10.1149/1.3535269)
Z. Lu, S. H. Lee, S. V. Babu, E. Matijević, J. Colloid Interface Sci. 55 (2003) 1 (https://doi.org/10.1016/S0021-9797(02)00166-2)
T. Du, D. Tamboli, V. Desai, V. S. Chathapuram, K. B. Sundaram, J. Mater. Sci. Mater. Electron. 87 (2004) 15 (https://doi.org/10.1023/B:JMSE.0000005381.96813.0f)
M. Christopher Sulym, D. Roy, Appl. Surf. Sci. 2583 (2010) 256 (https://doi.org/10.1016/j.apsusc.2009.10.108)
N. H. Kim, J. H. Lim, S. Y. Kim, E. G. Chang, Mater. Lett. 4601 (2003) 57 (https://doi.org/10.1016/S0167-577X(03)00368-9)
P. W. Carter, J. Zhang, J. Wang, S. Li, J. Electrochem. Soc. H378 (2008) 155 (https://doi.org/10.1149/1.2898683)
A. Jindal, Y. Li, S. V. Babu, Mater. Res. Soc. Symp. Proc. 8 (2001) 671 (https://doi.org/10.1149/1.1792871)
S. V. S. B. Janjam, S. Peddeti, D. Roy, S.V. Babu, Electrochem. Solid-State Lett. H327 (2008) 11 (https://doi.org/10.1149/1.2980345)
V. B. Kaufman, R. C. Kistler, S. Wang, (Cabot Corporation) US006063306A (2000)
F. Altaf, R. Qureshi, S. Ahmed, A. Y. Khan, A. Naseer, J. Electroanal. Chem. 642 (2010) 98 (https://doi.org/10.1016/j.jelechem.2010.02.011)
A. Shukla, S. N. Victoria, R. Manivannan, J. Indian Chem. Soc. 97 (2020) 1021 (https://indianchemicalsociety.com/portal/uploads/journal/2020_07_11_Extended_1605511486.pdf)
A. Jindal, S. V. Babu, J. Electrochem. Soc. G709 (2004) 151 (https://doi.org/10.1149/1.1792871)
S. Kim, N. Saka, J. H. Chun, Procedia CRIP 42 (2014) 14 (https://doi.org/10.1109/TSM.2014.2335156)
K. Yadav, M. Ramachandran, S.N. Victoria, ECS Trans. 59 (2018) 6 (https://doi.org/10.1149/08506.0059ecst)
H. Yang, S. Yang, Y. Cai, G. Hou, M. Tang, Electrochim. Acta 2829 (2010) 55 (https://doi.org/10.1016/j.electacta.2009.12.074)
G. J. Brug, A. L. G. van den Eeden, M. Sluyters-Rehbach, J. H. Sluyters, J. Electroanal. Chem. Interf. Electrochem. 275 (1984) 176 (https://doi.org/10.1016/S0022-0728(84)80324-1)
R. P. Venkatesh, S. Ramanathan, J. Appl. Electrochem. 767 (2010) 40 (https://doi.org/10.1007/s10800-009-0055-4)
K. Yadav, R. Manivannan, S. N. Victoria, ECS J. Solid State Sci. Technol. P879 (2017) 6 (https://doi.org/10.1149/2.0301712jss)
K. Yadav, R. Manivannan, S. N. Victoria, Mater. Today Proc. 1220 (2019) 18 (https://doi.org/10.1016/j.matpr.2019.06.584)
A. Robin, J. Appl. Electrochem. 37 (2003) 33 (https://doi.org/10.1023/A:1022982320438)
T. Du, J. Chen, D. Cao, J. Mater. Sci. 3903 (2001) 36 (https://doi.org/10.1023/A:1017909919388)
M. Pourbaix, Mater. Sci. Forum 43 (1974) http://sunlight.caltech.edu/aic/pourbaix.pdf
Y. H. Chen, T. H. Tsai, S. C. Yen, Microelectron. Eng. 174 (2010) 87 (https://doi.org/10.1016/j.mee.2009.07.009)
M. C. Turk, S. E. Rock, H. P. Amanapu, L. G. Teugels, D. Roy, ECS J. Solid State Sci. Technol. P205 (2013) 5 (https://doi.org/10.1149/2.009305jss).