Evaluation of derivatives of 2,3-dihydroquinazolin-4(1H)-one as inhibitors of cholinesterases and their antioxidant activity: In vitro, in silico and kinetics studies Scientific paper
Main Article Content
Abstract
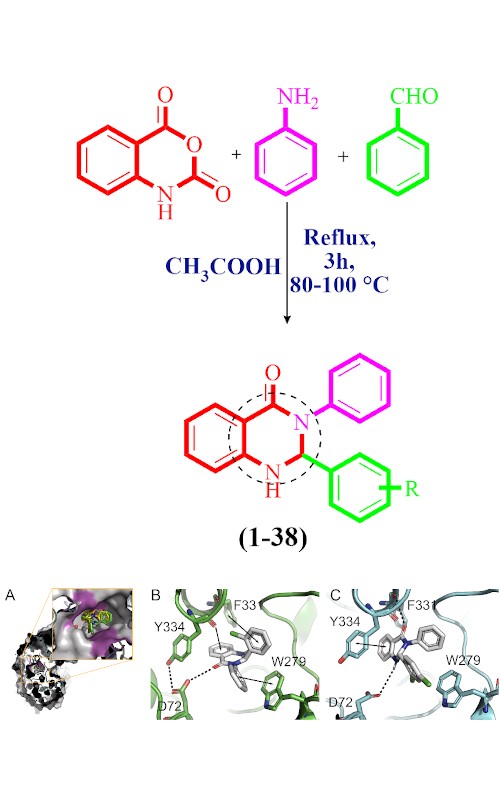
In search of potent inhibitors of cholinesterase enzymes and antioxidant agents, synthetic derivatives of dihydroquinazolin-4(1H)-one (1–38) were evaluated as potential anti-Alzheimer agents through in vitro acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitions and radical (DPPH and ABTS) scavenging activities. The structure–activity relationship (SAR) was mainly based on the different substituents at the aryl part which showed a significant effect on the inhibitory potential of enzymes and radical scavenging activities. The kinetic studies of most active compounds showed a noncompetitive mode of inhibition for AChE and a competitive mode of inhibition for the BChE enzyme. Additionally, molecular modelling studies were carried out to investigate the possible binding interactions of quinazolinone derivatives with the active site of both enzymes.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Higher Education Commission, Pakistan
Grant numbers SHEC/SRSP/Med-3/15/2021-21
References
S. Kumar, D. S. Brijeshlata, S. Dixit, Int. J. Pharm. Bio. Sci. 3 (2012) 59 (https://www.mendeley.com/catalogue/e895c115-5ca8-3d1b-956e-0477555aecc6/Int-J-Pharma-Bio-Sci)
B. Desgranges, J. C. Baron, V. de la Sayette, M. C. Petit-Taboue, K. Benali, B. Landeau, F. Eustache, J. Neurol. 121 (1998) 611 (https://doi.org/10.1093/brain/121.4.611)
R. M. Lane, S. G. Potkin, A. Enz, Int. J. Neuropsychopharmacol. 9 (2006) 101 (https://doi.org/10.1017/S1461145705005833)
T. Zhao, K. M. Ding, L. Zhang, X. M. Cheng, C. H. Wang, Z. T. Wang, J. Chem. 2013 717232 (https://doi.org/10.1155/2013/717232)
H. Guo, S. Albrecht, M. Bourdeau, T. Petzke, C. Bergeron, A. C. LeBlanc, Am. J. Clin. Pathol. 165 (2004) 523 (https://doi.org/10.1016/S0002-9440(10)63317-2)
S. Kumar, Ind. J. Pharmacol. 47 (2015) 444 (https://doi: 10.4103/0253-7613.161274)
N. H. Greig, D. K. Lahiri, K. Sambamurti, Int. Psychogeriatr. 14 (2002) 77 (https://doi.org/10.1017/S1041610203008676)
M. Asif, Int. J. Med. Chem. 2014 395637 (https://doi.org/10.1155/2014/395637)
E. Jafari, M. R. Khajouei, F. Hassanzadeh, G. H. Hakimelahi, G. A. Khodarahmi, Res. Pharm. Sci. 11 (2016) 1 (https://www.mendeley.com/catalogue/fde6c1f7-4ecf-310e-b761-c9a2f037543e/Res-Pharm-Sci)
S. K. Wahan, B. Sharma, P. A. Chawla, J. Heterocycl. Chem. 59 (2022) 239 (https://doi.org/10.1002/jhet.4382)
D. Wang, F. Gao, Chem. Cent. J. 7 (2013) 1 (https://doi.org/10.1186/1752-153X-7-95)
P. S. Auti, G. George, A. T. Paul, RSC Adv. 10 (2020) 41353 (https://doi.org 10.1039/D0RA06642G)
S. S. AlNeyadi, N. Amer, T. G. Thomas, R. Al Ajeil, P. Breitener, N. Munawar, Heterocycl. Comm. 26 (2020) 112 (https://doi.org/10.1515/hc-2020-0112)
K. N. Mohana, C. B. P. Kumar, Int. Scholarly Res. Notices 2013 620718 (https://doi.org/10.1155/2013/620718)
F. Z. Fang, S. Yang, G. Wu, Nutr. 18 (2002) 872 (https://doi.org/10.1016/S0899-9007(02)00916-4)
S. Cuzzocrea, D. P. Riley, A. P. Caputi, D. Salvemini, Pharmacol. Rev. 53 (2001) 135 (https://www.mendeley.com/catalogue/a54c6abb-aa6e-343f-91cf-06f546524153/ Pharmacol-Rev)
A. Choudhary, R. Sharma, M. Nagar, M. Mohsin, H. S. Meena, J. Chil. Chem. Soc. 56 (2011) 911 (http://dx.doi.org/10.4067/S0717-97072011000400019)
F. Rahim, M. T. Javed, H. Ullah, A. Wadood, M. Taha, M. Ashraf, K. M. Khan, Bioorg. Chem. 62 (2015) 106 (https://doi.org/10.1016/j.bioorg.2015.08.002)
M. A. Abbasi, M. Ilyas, A. Sonia, D. Shahwar, M. A. Raza, K. M. Khan, N. Ambreen, Sci. Iran 19 (2012) 1580 (https://doi.org/10.1016/j.scient.2012.10.014)
K. Mohammed Khan, U. Rasool Mughal, N. Ambreen, N. Hasan Rama, F. Naz, S. Perveen, M. Iqbal Choudhary, Lett. Drug Des. Discov. 7 (2010) 716 (https://www.mendeley.com/catalogue/e16f7904-d62c-3e15-9d04-773c212f8746/Lett-Drug-Des-Discov)
K. Mohammed Khan, M. Rani, N. Ambreen, A. Ejaz, S. Perveen, S. Moazzam Haider, W. Voelter, Lett. Drug Des. Discov. 9 (2012) 135 (https://www.mendeley.com/catalogue/42ad6641-237f-3505-b9af-9360184eee91/Lett-Drug-Des-Discov)
K. Javaid, S. M. Saad, S. Rasheed, S. T. Moin, N. Syed, I. Fatima, M. Choudhary, M, Bioorg. Med. Chem. 23 (2015) 7417 (https://doi.org/10.1016/j.bmc.2015.10.038)
O. Babatunde, S. Hameed, U. Salar, S. Chigurupati, A.Wadood, A. U. Rehman, S. Perveen, Mol. Divers. (2021) 1 (https://doi.org/10.1007/s11030-021-10196-5).
K. M. Khan, S. M. Saad, N. N Shaikh, S. Hussain, M. I Fakhri, S. Perveen, S. M. I. Choudhary, Bioorg. Med. Chem. 22 (2014) 3449 (https://doi.org/10.1016/j.bmc.2014.04.039)
S. Perveen, S. M. Saad, S. Perveen, A. Hameed, M. T. Alam, K. M. Khan, M. I. Choudhary, J. Chem. Soc. Pak. 38 (2016) (https://www.mendeley.com/catalogue/ecd7844f-d565-3fcd-8bb0-28a836aefd53/J-Chem-Soc-Pak)
G. L. Ellman, K. D. Courtney, Jr. V. Andres, R. M. Featherstone, Biochem. Pharmacol. 7 (1961) 88 (https://doi.org/10.1016/0006-2952(61)90145-9)
P. Molyneux, Songklanakarin, J. Sci. Technol. 26 (2004), 211
M. S. Blois, Nature 181 (1958) 1199 (https://doi.org/10.1038/1811199a0)
U. Kulsoom, U. Salar, K. M. Khan, S. Chigurupati, S. Syed, A. Wadood, A. Ur Rehman, B. Fatima, F. Saleem, M. Taha, S. G. Felemban, S. R. Dachani, S. Perveen, Monatsh. Chem. 153 (2022) 949 (https://doi.org/10.1007/s00706-022-02972-2)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. 26 (1999) 1231 (https://doi.org/10.1016/S0891-5849(98)00315-3)
Molecular Operating Environment (MOE), 2016.08; Chemical Computing Group Inc., Montreal, QC, 2016 (https://www.mendeley.com/catalogue/89d1dadd-e734-39ed-beda-f3d9995d868b/Montreal-QC-Canada)
S. Di Giovanni, A. Borloz, A. Urbain, A. Marston, K. Hostettmann, P. A. Carrupt, M. Reist, Eur. J. Pharm. Sci. 33 (2008) 109 (https://doi.org/10.1016/j.ejps.2007.10.004).