Electrical conductivity of GdCl3–LiCl and GdCl3–LiCl-Gd2O3 molten systems Scientific paper
Main Article Content
Abstract
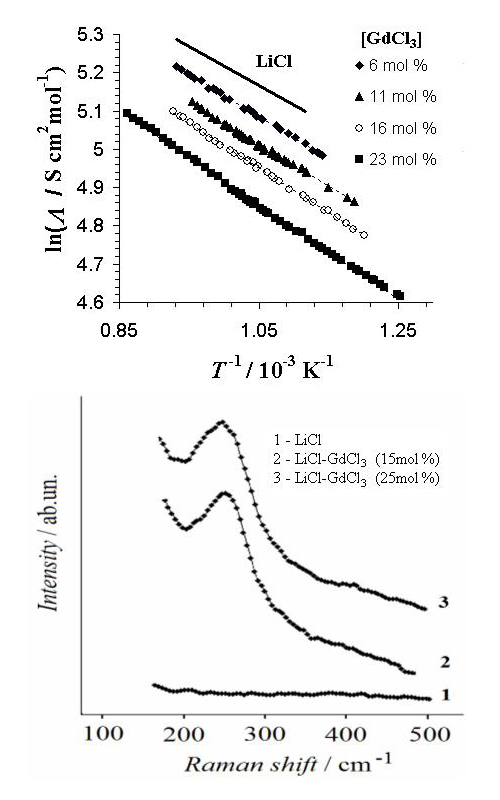
The electrical conductivity of LiCl–GdCl3 molten systems with the gadolinium chloride additions ranging from 0 to 23 mol % was measured depending on both the temperature and concentration of GdCl3. The molar conductivity of the molten GdCl3–LiCl system is calculated taking into account the assumption of additivity of the molar volume of the mixture. The obtained temperature dependencies can be approximated by Arrhenius-type equation. The effective activation energy, Ea, increased with the GdCl3 content. The liquidus temperatures of the studied systems were determined by differential scanning calorimetry. The high-temperature Raman spectra of LiCl–GdCl3 chloride melts were recorded. In addition, the conductivity of 0.77LiCl–0.23GdCl3 molten system with 1 mol % of Gd2O3 was measured. The investigation demonstrates that the addition of gadolinium oxide results in a decrease of the conductivity of the chloride molten system and growth of its liquidus temperature.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
T. Koyama, M. Fujita, M. Iizuka, Y. Sumida, Nucl. Technol. 110 (1995) 357 (https://doi.org/10.13182/NT95-A35107)
J. J. Laidler, J. E. Battles, W. E. Miller, J. P. Ackerman, E. L. Carls, Prog. Nucl. Energy 31 (1997) 131 (https://doi.org/10.1016/0149-1970(96)00007-8)
C. E. Till, Y. I. Chang, in Advances in Nuclear Science and Technology, Vol. 20, J. Lewins, M. Becker, Eds., Springer, Boston, MA., 1988, pp.127–154 (https://doi.org/10.1007/978-1-4613-9925-4_3)
G.-Y. Kim, J. Jang, S. Paek, S.-J. Lee, Sci. Tech. Nucl. Instal. (2020) 2392489 (https://doi.org/10.1155/2020/2392489)
G. J. Janz, J. Phys. Chem. Ref. Data 17 (1988) 3 (https://srd.nist.gov/JPCRD/jpcrdS2Vol17.pdf)
Potapov, L. Rycerz, M. Gaune-Escard, in Proceedingsof the International Symposium on Ionic Liquids, 2003, Carry le Rouet, France, International Symposium on Ionic Liquids in honour of Professor Marcelle Gaune-Escard, Trondheim, Norway, 2003, p.469
K. Fukushima, M. Hayakawa, Y. Iwadate, J. Alloys Comp. 245 (1996) 66 (https://doi.org/10.1016/S0925-8388(96)02485-1)
Y. Shuyun, Y. Weiqian, Y. Yuntao, S. Yunfu, T. Dingxiang, Chin. J. Appl. Chem. 1 (1984) 21 (http://yyhx.ciac.jl.cn/EN/Y1984/V0/I1/21)
E. V. Nikolaeva, I. D. Zakir’yanova, A.L. Bovet, T. V. Sosnovtseva, Rus. Metal. (Metally) 8 (2020) 817 (https://doi.org/10.1134/S003602952008011X)
E. V. Nikolaeva, I. D. Zakiryanova, A. L. Bovet, J. Electrochem. Soc. 169 (2022) 036511 (https://doi.org/10.1149/1945-7111/ac5b37)
W. Zhou, Y.Wang, J. Zhang, M. Khafizovet, J. Nucl. Mat. 508 (2018) 40 (https://doi.org/10.1016/j.jnucmat.2018.05.030)
I. V. Korzun, I. D. Zakiryanova, E. V. Nikolaeva, Rus. Metal. (Metally) 3 (2018) 271 (https://doi.org/10.1134/s0036029518080104)
I. Barin, G. Platzky, Thermochemical Data of Pure Substances, 3rd ed., Wiley, 1995, p. 956 (ISBN 9780471188155)
I. Barin, F. Sauert, E. Schultze-Rhonhof, W. S. Sheng, Thermochemical data of pure substances I, VCH Verlagsgesellschaft, Weinheim/VCH Publishers, New York 1989, p. 753 (ISBN 9780895738660)
H. J. Seifert, J. Therm. Anal. Calorim. 82 (2005) 575 (https://doi.org/10.1007/s10973-005-6946-7)
L. Rycerz, M. Gaune-Escard, J. Alloy Compd. 450 (2008) 167 (https://doi.org/10.1016/j.jallcom.2006.12.096)
R. J. M. Konings, A. Kovacs, in Handbook on physics and chemistry of rare earths, Vol. 33, K.A. Gschneidner Jr., J.-C.G. Bünzli, V.K. Pecharsky, Eds., Springer, New York, 2003 pp. 147–247 (https://doi.org/10.1016/S0168-1273(02)33003-4)
E. V. Nikolaeva, I. D. Zakiryanova, A. L. Bovet, I. V. Korzun, J. Electrochem. Soc. 168 (2021) 016502 (https://doi.org/10.1149/1945-7111/abd64a)
L. Rycerz, J. Therm Anal. Calorim. 113 (2013) 231 (https://doi.org/10.1007/s10973-013-3097-0)
V. M. Minchenko, V. P. Stepanov, Ionic melts: tensive and caloric properties, IHTE UB RAS, Yekaterinburg, 2008, pp. 77–82 (http://www.ihte.uran.ru/?page_id=3817)
K. Cho, K. Irisawa, J. Mochinaga, T. Kuroda, Electrochim. Acta 17 (1972) 10 (https://doi.org/10.1016/0013-4686(72)85073-4)
H. Tatlipinar, Z. Akdeniz, G. Pastore, M. P. Tosi, J. Phys. Condens. Matter. 4 (1992) 8933 (https://doi.org/10.1088/0953-8984/4/46/001)
J. Mochinaga, M. Ikeda, K. Igarashi, K. Fukushima, Y. Iwadate, J. Alloys Comp. 193 (1993) 36 (https://doi.org/10.1016/0925-8388(93)90301-3)
G. N. Papatheodorou, S. N. Yannopoulos, in NATO Science Series II, Molten Salts:From Fundamental to Applications, M. Gaune-Escard, Ed., Kluwer Academic Publishers, Dordrecht, 2002, p. 47 (https://doi.org/10.1007/978-94-010-0458-9)
Y. Okamoto, H. Shiwaku, T. Yaita, S. Suzuki, M. Gaune-Escard, J. Mol. Liq. 187 (2013) 94 (https://doi.org/10.1016/j.molliq.2013.05.018)
M. Zabłocka-Malicka, W. Szczepaniak, J. Mol. Liq. 137 (2008) 36 (https://doi.org/10.1016/j.molliq.2007.03.004)
M. Zabłocka-Malicka, B. Ciechanowski, W. Szczepaniak, W. Gaweł, Electrochim. Acta 53 (2008) 2081 (https://doi.org/10.1016/j.electacta.2007.08.073)
R. D. Shannon, Acta Cryst. 32 (1976) 751 (http://dx.doi.org/10.1107/S0567739476001551)
D. Zakiryanov, Comput. Theor. Chem. 1210 (2022) 113646 (https://doi.org/10.1016/j.comptc.2022.113646)
S. A. Kirillov, Rus. J. Electrochem. 47 (2007) 901 (https://doi.org/10.1134/S1023193507080083)
I. D. Zakiryanova, D. O. Zakiryanov, P. O. Zakiryanov, J. Mol. Liq. 376 (2023) 121485 (https://doi.org/10.1016/j.molliq.2023.121485).