Physicochemical properties of the heterogeneous system Li2CO3–Na2CO3–K2CO3/MgO Scientific paper
Main Article Content
Abstract
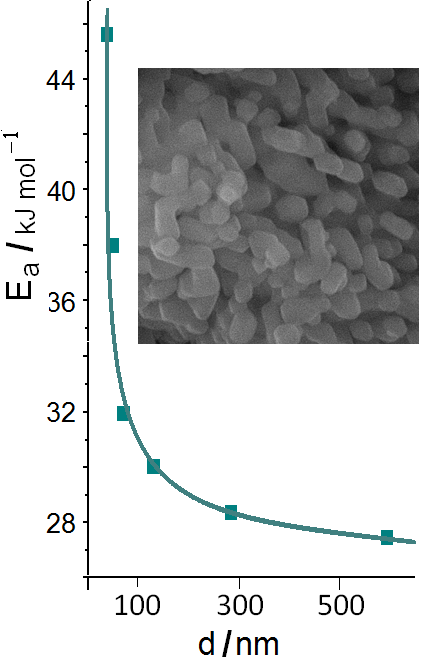
The structure, conductivity, melting points and caloric melting effects of the (Li2CO3–Na2CO3–K2CO3)eut melt/MgO nanopowder heterogeneous system with MgO concentration up to 70 vol. % have been investigated. A wide variety of methods (DSC, XRD, BET, high resolution scanning electron microscope, AC impedance method, IR and Raman spectroscopy) were used to evaluate samples and research. It is revealed that at the values of effective thickness of the salt phase interlayer between MgO particles below 100 nm there is an abrupt decrease in the melting points of the salt and the normalized phase transition enthalpy of the heterogeneous system. The activation energy of the electrical conductivity rises as the values of effective thickness of the melt phase interlayer between MgO particles decreases. The study established the lack of any chemical interaction between MgO and carbonate melt at 400–600 °С. In situ Raman spectroscopy of the (Li2CO3–Na2CO3–K2CO3)eut melt/MgO nanopowder systems revealed the solvation of solid particles by salt-melt ions.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
R. Remick, D. Wheeler, Molten Carbonate and Phosphoric Acid Stationary Fuel Cells: Overview and Gap Analysis, National Renewable Energy Laboratory, Golden, CO, 2010 (https://www.nrel.gov/docs/fy10osti/49072.pdf)
M.W. Breiter, in Electrochemical Processes in Fuel Cells, Ed. M. Becke-Goehring, Springer, Berlin, 1969, p. 217 (https://doi.org/10.1007/978-3-642-46155-2_13)
S. Scaccia, J. Mol. Liquids 116 (2005) 67 (https://doi.org/10.1016/j.molliq.2004.07.078)
A. Kulkarni, S. Giddey, J. Solid State Electrochem. 16 (2012) 3123 (https://doi.org/10.1007/s10008-012-1771-y)
E. Antolini, Ceram. Int. 39 (2013) 3463 (https://doi.org/10.1016/j.ceramint.2012.10.236)
Zakir’yanova, I. Korzun, V. Khokhlov, V. Dokutovich, E. Nikolaeva, B. Antonov, Russ. J. Appl. Chem. 89 (2016) 1066 (https://doi.org/10.1134/S1070427216070041)
V. Dokutovich, V. Khokhlov, I. Zakir’yanova, Int. J. Heat Mass Trans. 119 (2018) 365 (https://doi.org/10.1016/j.ijheatmasstransfer.2017.11.132)
E. Nikolaeva, A. Bovet, I. Zakiryanova, Zeitschrift Naturforsch., A 73 (2018) 79 (https://doi.org/10.1515/zna-2017-0222)
M. Mizuhata, T. Ohashi, A. Béléké, Int. J. Hydrogen Energy 37 (2012) 19407 (https://doi.org/10.1016/j.ijhydene.2011.09.109)
M. Mizuhata, Y. Harada, G. Cha, A. Béléké, S. Deki, J. Electrochem. Soc. 151 (2004) 179 (https://doi.org/10.1149/1.1688798 )
M. Mizuhata, A. Béléké, H. Watanabe, Y. Harada, S. Deki, Electrochim. Acta 53 (2007) 71 (https://doi.org/10.1016/j.electacta.2007.06.020)
R. Jenkins, R. Snyder, Introduction to X-ray Powder Diffractometry, John Wiley & Sons, New York, 1996 (https://doi.org/10.1002/9781118520994)
I. Zakir`yanova, E. Nikolaeva, A. Bove, J. Appl. Spectrosc. 81 (2015) 919 (https://doi.org/10.1007/s10812-015-0029-8)
I. Zakir′yanova, P. Arkhipov, D. Zakir′yanov, J. Appl. Spectrosc. 82 (2016) 920 (https://doi.org/10.1007/s10812-016-0205-5
G. J. Janz, J. Phys. Chem. Ref. Data 17 (1988) 3 (https://srd.nist.gov/JPCRD/jpcrdS2Vol17.pdf)
A. Ulihin, N. Uvarov, ECS Trans. 16 (2009) 445 (https://doi.org/10.1149/1.3242260)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, John Wiley & Sons, New York, 2009 (https://doi.org/10.1002/9780470405840)
I. D. Zakir′yanova, J. Appl. Spectr., 85 (2018) 611 (https://doi.org/10.1007/s10812-018-0694-5)
Béléké, M. Mizuhata, S. Deki. Vibr. Spectrosc. 40 (2006) 66 (https://doi.org/10.1016/j.vibspec.2005.07.002).