The lithium oxide solubility in molten fluoride system CeF3 – FLiNaK Scientific paper
Main Article Content
Abstract
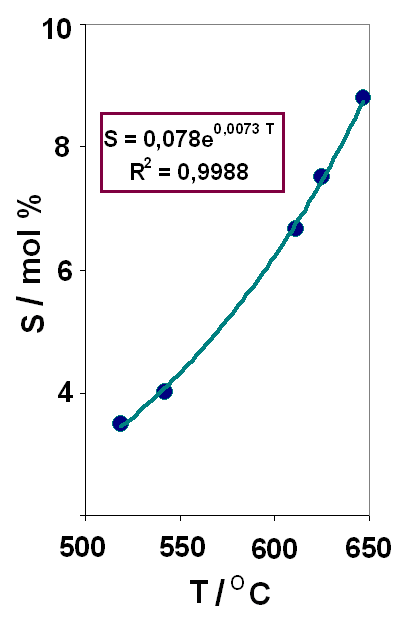
Molten systems based on alkali metal halides with lithium oxide additives are promising as a working medium on pyrochemical reprocessing of nuclear waste. A mixture of alkali metal fluorides of eutectic composition (FLiNaK) is a suggested solvent due to the high solubility of actinide oxides, low viscosity, high boiling points, low vapor pressure, and resistance to radiation damage. Thermal analysis, XRD, Raman spectroscopy and thermodynamic simulations were used to obtain evidences on the phase equilibria and liquidus points of the system (0.85 FLiNaK – 0.15 CeF3) – Li2O, containing up to 8.8 mol% lithium oxide. The solubility of lithium oxide in the fluoride melt FLiNaK – CeF3 and the thermodynamic parameters of dissolution were obtained. The eutectic point (the Li2O content is 3.1 mol%, Tm = 489 ºС) and two peritectic points (lithium oxide content are 3.2 and 4.2 mol%, and liquidus points are 497 and 549 ºС, respectively) were found. Thermodynamic simulation results show an exothermic effect due to interaction between lithium oxide and fluoride melt. The interaction product oxyfluoride CeOF was detected by XRD analysis and Raman spectroscopy.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. Lee, G.-I. Park, K.-H. Kang, J.-M. Hur, J.-G. Kim, D.-H. Ahn, Y.-Z. Cho, E. H. Kim, Nucl. Eng. Tech. 43 (2011) 317 (https://doi.org/10.5516/NET.2011.43.4.317)
S. M. Jeong, B.H. Park, J.-M. Hur, C.-S. Seo, H. Lee, K.-C. Song, Nucl. Eng. Tech. 42 (2010) 183 (https://doi.org/10.5516/NET.2010.42.2.183)
E.-Y. Choi, C. Y. Won, D.-S.Kang, S.-W. Kim, J. Radioanal. Nucl. Chem. 304 (2015) 535 (https://doi.org/10.1007/s10967-014-3842-2)
Y. P. Zaikov, V. Y. Shishkin, A. M. Potapov, A. E. Dedyukhin, J. of Physics: Conf. Series 1475 (2020) 012027 (https://doi.org/10.1088/1742-6596/1475/1/012027)
M. Iizuka, T. Inoue, M. Ougier, J.-P. Glatz, J. Nucl. Sci. Tech. 44 (2007) 801 (https://doi.org/10.1080/18811248.2007.9711869)
B. H. Park, I. W. Lee, C. S. Seo, Chem. Eng. Sci. 63 (2008) 3485 (https://doi.org/10.1016/j.ces.2008.04.021)
D. Kim, S. Bae, J. Kim, T. Park, Y. Park, K. Song, Asian J. Chem. 25 (2013) 705 (http://dx.doi.org/10.14233/ajchem.2013.18)
Y. Kado, T. Goto, R. Hagiwara, J. Chem. Eng. Data 53 (2008) 2816 (https://doi.org/10.1021/je800540c)
A. Mullabaev, O. Tkacheva, V. Shishkin, V. Kovrov, Y. Zaikov, L. Sukhanov, Y. Mochalov, J. Nucl. Mat. 500 (2018) 235 (https://doi.org/10.1016/j.jnucmat.2018.01.004)
R. G. Reddy, S. G. Kumar, Metal. Trans. B 24B (1993) 1031 (https://doi.org/10.1007/BF02660994)
A. A. Maslennikova, P. N. Mushnikov, A. V. Dub, O. Y. Tkacheva, Y. P. Zaikov, Y.-L. Liu, W.-Q. Shi, Materials 16 (2023) 4197 (https://doi.org/10.3390/ma16114197)
X. Guo, J. Sietsma, Y. Yang, A Critical Evaluation of Solubility of Rare Earth Oxides in Molten Fluorides, in Rare Earths Industry, Eds.: I. B. De Lima, W. L. Filho, Elsevir, 2016, p. 223 (https://doi.org/10.1016/B978-0-12-802328-0.00015-2)
A. L. Rollet, E. Veron and C. Bessada, J. Nucl. Mater. 429 (2012) 40 (https://doi.org/10.1016/j.jnucmat.2012.05.010)
O. Beneš, R. J. M. Konings, J. Nucl. Mater. 435 (2013) 164 (https://doi.org/10.1016/j.jnucmat.2012.12.005)
R. Marsac, F. Réal, N. Banik, M. Pédrot, O. Pourret, V. Vallet, Dalton Trans. 46 (2017) 13553 (https://doi.org/10.1039/C7DT02251D)
D. Zakiryanov, J. Mol. Liq. 384 (2023) 122265 (https://doi.org/10.1016/j.molliq.2023.122265)
D. Zakiryanov, Molecular Simulation (2023) 845 (https://doi.org/10.1080/08927022.2023.2193656)
HSC Chemistry 9 [Software], Outokumpu Research Oy: Pori, Finland, 2007.
G. Zong, Z. Zhang, J. Sun, J. Xiao, J. Fluor. Chem. 197 (2017) 134 (https://doi.org/10.1016/j.jfluchem.2017.03.006)
J. Derek, Y. Toshinobu, J. Janz, J. Chem. Eng. Data 27 (1982), 366 (https://doi.org/10.1021/je00029a041)
R. P. Bauman, S. P. S. Porto, Phys. Rev. 161 (1967) 842 (https://doi.org/10.1103/PhysRev.161.842)
P. N. Mushnikov, O. Y. Tkacheva, A. S. Kholkina, Y. P. Zaikov, V. Y. Shishkin, A. V Dub, At. Energy 131 (2022) 263 (https://doi.org/10.1007/s10512-022-00876-2)
F. S. Gittleson, K. Yao, D. G. Kwabi, S. Y. Sayed, W. Ryu, Y. Shao-Horn, A. D. Taylor, Chem Electro Chem. 2 (2015) 1446 (https://doi.org/10.1002/celc.201500218)
T. Osaka, I. Shindo, Solid State Commun. 51 (1984) 421 (https://doi.org/10.1016/0038-1098(84)90126-1)
E. M. Rodrigues, E. R. Souza, J. K. Monteiro, R. D. L. Gaspar, I. O. Mazali, F. A. Sigoli, J. Mater. Chem. 22 (2012) 24109 (https://doi.org/10.1039/c2jm34901a)
J. Hölsä, E. Säilynoja, H. Rahiala, J. Valkonen, Polyhedron 16 (1997) 3421 (https://doi.org/10.1016/S0277-5387(97)00065-X)
E. V. Nikolaeva, I. D. Zakiryanova, A. L. Bovet, I. V. Korzun, Z. Naturforsch. 71 (2016) 731 (https://doi.org/10.1515/zna-2016-0163)
E. V. Nikolaeva, I. D. Zakiryanova, I. V. Korzun, A. L. Bovet, B. D. Antonov, Z. Naturforsch. 70 (2015) 325 (https://doi.org/10.1515/zna-2014-0370).