Assessing the pharmacological potential of selected xanthene derivatives Scientific paper
Main Article Content
Abstract
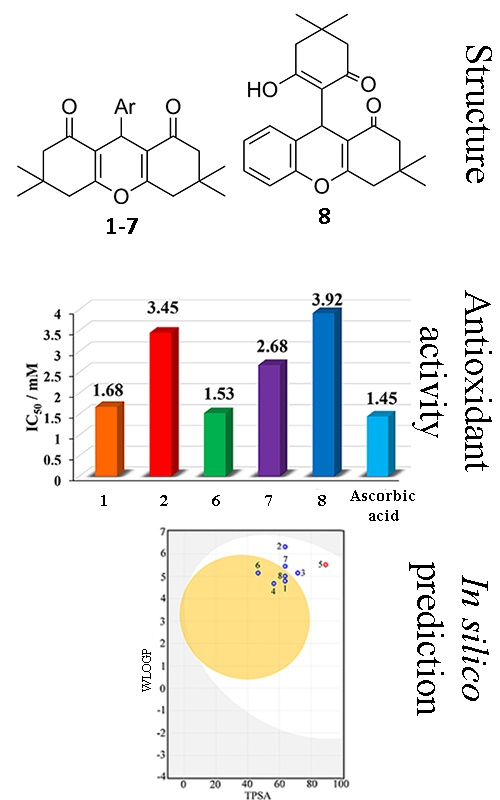
A convenient and efficient approach toward the synthesis of seven aromatically substituted xanthendiones 1‒7 and one structurally-related xanthenone 8 through condensation of dimedone and the appropriate aromatic aldehyde is reported. Further, their chemical structure was confirmed by melting points, elemental analysis, FT-IR, 1H-, 13C-NMR and UV–Vis spectroscopic methods. The relationship between the chemical structure and pharmacological activity was determined empirically using appropriate software packages and in vitro using the 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method. The results of in silico prediction suggested that all investigated compounds possess good oral bioavailability. The results of the ABTS assay indicate that five compounds possess the ability to scavenge the ABTS•+ radical cation. Based on the comparison of the IC50 values, the activity of the compounds was found to be as follows: 6 > 1 > 7 > 2 > 8. The effects of solvent dipolarity/polarizability and solute solvent–hydrogen-bonding interactions on the shifts of the absorption maxima were rationalized by means of the linear solvation energy relationship concepts proposed by Kamlet–Taft and Catalán.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200135;451-03-68/2022-14/200287
References
N. Karmaker, D. N. Lira, B. K. Das, U. Kumar, A.S. S. Rouf, Dhaka Univ. J. Pharm. Sci. 16 (2017) 245 (https://dx.doi.org/10.3329/dujps.v16i2.35263)
T. K. Khatab, A. El-Mekabaty, Z. M. Gamala, E. M. Kandil, Egypt. J. Chem. 61 (2018) 661 (https://dx.doi.org/10.21608/ejchem.2018.3381.1285)
A. G. Ghahsare, Z. S. Nazifi, S. M. R. Nazifi, Curr. Org. Synth. 16 (2019) 1071 (https://dx.doi.org/10.2174/1570179416666191017094908)
W. A. A. Fadaly, Y. A. M. M. Elshaier, M. T. M. Nemr, K. R. A. Abdellatif, Bioorg. Chem. 134 (2023) 106428 (https://dx.doi.org/10.1016/j.bioorg.2023.106428)
M. T. M. Nemr, A.M. AboulMagd, Bioorg. Chem. 103 (2020) 104134 (https://dx.doi.org/10.1016/j.bioorg.2020.104134)
A. H. M. Hussein, A. A. Khames, A.-B. A El-Adasy, A. A. Atalla, M. Abdel-Rady, M. I. A. Hassan, M. T. M. Nemr, Y. A. A. M.Elshaier, RSC Adv. 10 (2020) 29723 (https://dx.doi.org/10.1039/d0ra05561a)
M. T. M. Nemr, A. Sonousi, A. A. Marzouk, Bioorg. Chem. 105 (2020) 104446 (https://dx.doi.org/10.1016/j.bioorg.2020.104446)
Shagufta, I. Ahmad, Eur. J. Med. Chem. 116 (2016) 267 (https://dx.doi.org/10.1016/j.ejmech.2016.03.058)
M. Maia, D. I. S. P. Resende, F. Durães, M. M. M. Pinto, E. Sousa, Eur. J. Med. Chem. 210 (2021) 113085 (https://dx.doi.org/10.1016/j.ejmech.2020.113085)
Í. E. Poly da Silva, M. Lopes da Silva, R. Sousa Dias, E. Gonçalves Santos, M. C. Brangioni de Paula, A. Silva de Oliveira, A. F. Costa da Silveira Oliveira, F. Marques de Oliveira, C. Canedo da Silva, R. R. Teixeira, S. Oliveira de Paula, Microbes Infect. 22 (2020) 489 (https://dx.doi.org/1016/j.micinf.2020.04.007)
E. Veljović, S. Špirtović-Halilović, S. Muratović, A. Osmanović, I. Novaković, S. Trifunović, D. Završnik, Bull. Chem. Technol. Bosnia Herzegovina 51 (2018) 13
S. Zukić, E. Veljović S. Špirtović-Halilović, S. Muratović, A. Osmanović, S. S. Trifunović, I. Novaković, D. Završnik, Croat. Chem. Acta 91 (2018) 1 (https://dx.doi.org/10.5562/cca3225).
R. Retnosari, K. K. Sari, S. Marfu’ah, Sutrisno, I. B. Rachman, Commun. Sci. Technol. 7 (2022) 181 (https://dx.doi.org/10.21924/cst.7.2.2022.963)
R. Retnosari, N. Ultiyati, A. Santoso, S. Marfu’ah, I. B. Rachman, J. Kim. Kemasan 43 (2021) 117 (https://dx.doi.org/10.24817/jkk.v43i2.7027)
A. H. Bhat, V. R. Shah, M. R. Rawal, World J. Pharm. Res. 118 (2019) 100 (https://dx.doi.org/10.20959/wjpr20179-9254)
A. P. de Jesus Menezes, M. Lopes da Silva, W. Luiz Pereira, G. de Paula Costa , A. Luciano Horta, A. Aparecida Santos Mendonça, A. C. Alvarenga Carneiro, D. M. Soares de Souza, R. Dias Novaes, R. R. Teixeira, A. Talvani, J. Glob. Antimicrob. Resist. 22 (2020) 466 (https://dx.doi.org/10.1016/j.jgar.2020.04.005)
M. Alagumuthu, A. Siva Kumar, P. S. Nigam, A. A. Napoleon, Anti-Cancer Agents Med. Chem. 20 (2020) 909 (https://dx.doi.org/10.2174/1871520620666200318094138)
S. Khandelwal, Y. K. Tailor, E. Rushell, M. Kumar, Green Approaches in Medicinal Chemistry for Sustainable Drug Design, Elsevier Inc., Amsterdam, 2020, pp. 245–352 (https://dx.doi.org/10.1016/B978-0-12-817592-7.00009-5)
M. A. Bhat, A. M. Naglah, S. Akber Ansari, H. M. Al-Tuwajiria, A. Al-Dhfyan, Molecules 26 (2021) 3667 (https://dx.doi.org/10.3390/molecules26123667)
M. T. M. Nemr, M. A. M. AboulMagd, H. M. Hassan, A. A. Hamed, M. I. A. Hamed, M. T. Elsaadi, RSC Adv. 11 (2021) 26241 (https://dx.doi.org/10.1039/D1RA05277B)
M. T. M. Nemr, M. N. M.Yousif, J. Barciszewski, Arch. Pharm. (Weinheim) 352 (2019) 1 (https://dx.doi.org/10.1002/ardp.201900062)
S. F. Zhou, W. Z. Zhong, Molecules 22 (2017) 1 (https://dx.doi.org/10.3390/molecules22020279)
M. Remko, M. Swart, F. M. Bickelhaupt, Bioorg. Med. Chem. 14 (2006) 1715 (https://dx.doi.org/10.1016/j.bmc.2005.10.020)
B. Kuhn, P. Mohr, M. Stahl, J. Med. Chem. 53 (2010) 2601 (https://dx.doi.org/10.1021/jm100087s)
G. M. Ghiandoni, E. Caldeweyher, Sci. Rep. 13 (2023) 4143 (https://dx.doi.org/10.1038/s41598-023-30089-x)
E. Khan, S. A. Khan, A.S hahzad, A. Noor, J. Chem. Crystallogr. 45 (2015) 238 (https://dx.doi.org/10.1007/s10870-015-0588-9)
H. M. Metwally, N. A. Khalaf, E. Abdel-Latif, M. A. Ismail, BMC Chem. 17 (2023) 1 (https://dx.doi.org/10.1186/s13065-023-00917-2)
R. Mishra, N. Kumar, N. Sachan, Mini. Rev. Med. Chem. 22 (2022) 1420 (https://dx.doi.org/10.2174/1389557521666211022145458)
N. Banjac, N. Trišović, Ž. Vitnik, V. Vitnik, N. Valentić, G. Ušćumlić, I. Juranić, Monatsh. Chem. 144 (2013) 1525 (https://dx.doi.org/10.1007/s00706-013-1052-1)
M. Bauer, A. Rollberg, A. Barth, S. Spange, Eur. J. Org. Chem. 26 (2008) 4475 (https://dx.doi.org/10.1002/ejoc.200800355)
A. M. Reeve, J. Chem. Educ. 92 (2015) 582 (https://dx.doi.org/10.1021/ed400457c)
M. Bayat, H. Imanieh, S. H. Hossieni, Chin. Chem. Lett. 20 (2009) 656 (https://dx.doi.org/10.1016/j.cclet.2008.12.050)
http://www.swissadme.ch/. Accessed 10.01.2023
https://preadmet.bmdrc.kr/. Accessed 10.01.2023
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. 26 (1999) 1231 (https://dx.doi.org/10.1016/s0891-5849(98)00315-3)
P. Paliwal, S. R. Jetti, A. Bhatewara, T. Kadre, S. Jain, ISRN Org. Chem. (2013) 1 (https://dx.doi.org/10.1155/2013/526173)
Y. A. A. M. Elshaier, M. T. M. Nemr, M. S. Refaey, W. A. A. Fadaly, A. Barakat, New J. Chem. 46 (2022) 13383 (https://dx.doi.org/10.1039/D2NJ00460G)
M. Udayakumar, J. Kothandapani, S. S. Ganesan, V. N. Sathiyamoorthy, S. M. Kumar, K. Byrappa, S. Thamotharan, J. Mol. Struct. 1133 (2017) 510 (https://dx.doi.org/10.1016/j.molstruc.2016.11.082)
M. M. Heravi, V. Zadsirjan, M. Mollaiye, M. Heydari, A. T. K. Koshvandi, Russ. Chem. Rev. 87 (2018) 553 (https://dx.doi.org/10.1070/rcr4780)
A. K. Ghoshe, V. N. Viswanadhan, J. J. Wendoloski, J. Comb. Chem. 1 (1999) 55 (https://dx.doi.org/10.1081/rrs-100107923)
A. Bogdanović, A. Lazić, S. Grujić, I. Dimkić, S. Stanković, S. Petrović, Arh. Hig. Rada Toksikol. 72 (2021) 70 (https://dx.doi.org/10.2478/aiht-2021-72-3483)
P. Z. Li, Z. Q. Liu, Tetrahedron 69 (2013) 9898 (https://dx.doi.org/10.1016/j.tet.2013.08.053)
M. Spiegel, Z. Sroka, Theor. Chem. Acc. 141 (2022) 1 (https://dx.doi.org/10.1007/s00214-022-02922-5)
M. A. Gouda, G. E. Abd El‐Ggani, M. A. Berghot, A. E. M. Khalil, J. Heterocycl. Chem. 56 (2019) 2036 (https://dx.doi.org/10.1002/jhet.3584)
M. Olszowy, Plant Physiol. Biochem. 144 (2019) 135 (https://dx.doi.org/1016/j.plaphy.2019.09.039)
G. L. Xi, Z. Q. Liu, J. Agric. Food Chem. 63 (2015) 3516 (https://dx.doi.org/10.1021/acs.jafc.5b00399)
T. Narsinghani, M. C. Sharma, S. Bhargav, Med. Chem. Res. 22 (2013) 4059 (https://dx.doi.org/10.1007/s00044-012-0413-3)
B. W. Domagalska, K. A. Wilk, S. Wysocki, Phys. Chem. Chem. Phys. 5 (2003) 696 (https://dx.doi.org/10.1039/b208125c)
S. M. Martinez Gomez, D. M. Alzate Sanchez, W. Rodríguez-Córdoba, C. A. Sierra, C. Ochoa-Puentes, Synth. Commun. 44 (2014) 648 (https://dx.doi.org/10.1080/00397911.2013.831903)
G. K. Verma, K. Raghuvanshi, R. K.Verma, P. Dwivedi, M. S. Singh, Tetrahedron 67 (2011) 3698 (https://dx.doi.org/10.1016/j.tet.2011.03.078)
T. Yempala, B. Sridhar, S. Kantevari, J. Chem. Sci. 127 (2015) 803 (https://dx.doi.org/10.1007/s12039-015-0835-9)
N. Friebe, K. Schreiter, J. Kübel, B. Dietzek, N. Moszner, P. Burtscher, A. Oehlke, S. Spange, New J. Chem. 39 (2015) 5171 (https://dx.doi.org/10.1039/c5nj00256g)
S. Hmuda, N. Trišović, J. Rogan, D. Poleti, Ž. Vitnik, V. Vitnik, N. Valentić, B. Božić, G. Ušćumlić, Monatsh. Chem. 145 (2014) 821 (https://dx.doi.org/10.1007/s00706-013-1149-6)
K.Hofmann, S. Brumm, C. Mende, K. Nagel, A. Seifert, I. Roth, D. Schaarschmidt, N. Lang, S. Spange, New J. Chem. 36 (2012) 1655 (https://dx.doi.org/10.1039/C2NJ40313G).