The synthesis, characterization, antioxidant and antimicrobial activity of some novel amides of the esters of substituted 1,4-dihydropyridines Scientific paper
Main Article Content
Abstract
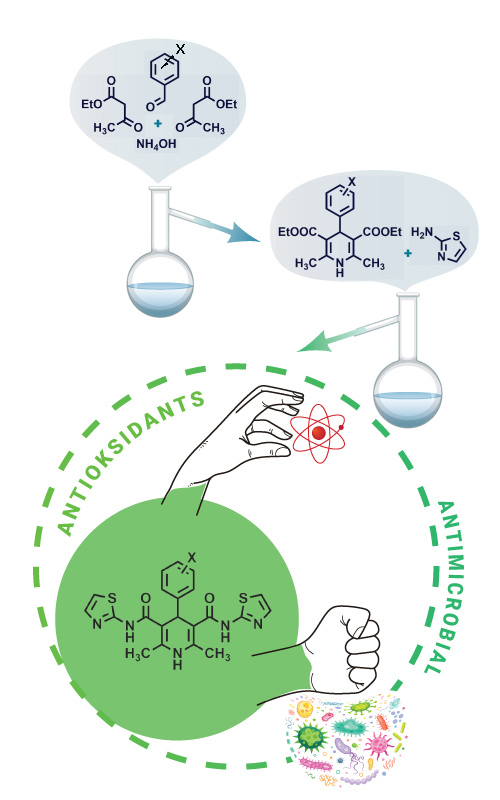
The esters of substituted 1,4-dihydropyirdines (1,4-DHP) are formed in the reaction of an appropriate aldehyde and ethyl acetoacetate in the presence of concentrated water solution of ammonia. The esters form the amides by the reaction with primary amines. The series of the amides has been synthesized with the aim to analyze their chemical characteristics, antioxidant and antimicrobial activity. The amine used in this research is 2-aminothiazole. The antioxidant activity is analysed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) methods and the antimicrobial activity screening was performed by broth microdilution method, using different microbial strains. The characterization of the obtained amides was done by melting points, FTIR, NMR and elemental analysis. The possibilities for further research was suggested, which could lead to the application of selected compounds.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200135
References
A. Debache, W. Ghalem, R. Boulcina, A. Belfaitah, S. Rhouati, B. Carboni, Tetrahedron Lett. 50 (2009) 5248 (https://doi.org/10.1016/j.tetlet.2009.07.018)
U. Eisner, J. Kuthan, Chem. Rev. 72 (1972) 1 (https://doi.org/10.1021/cr60275a001)
A. Velena, N. Zarkovic, K. Gall Troselj, E. Bisenieks, A. Krauze, J. Poikans, G. Duburs, Oxid. Med. Cell. Longev. 2016 (2016) (https://doi.org/10.1155/2016/1892412)
Y. Wei1 , Y. Lu, Y. Zhu, W. Zheng, F. Guo, Be. Yao, S. Xu, Y. Wang, L. Jin , Y. Li, Biochim. Biophys. Acta – Gen. Subj. 1862 (2018) 2261 (https://doi.org/10.1016/j.bbagen.2018.07.022)
E. D. Funder, J. B. Trads, K. V. Gothelf, Org. Biomol. Chem. 13 (2015) 185 (https://doi.org/10.1039/C4OB01931H)
A. E. Sausins, G. Duburs, Chem. Heterocycl. Compd. 28 (1992) 363 (https://doi.org/10.1007/bf00766993)
A. Kumar, R. A. Maurya, S. Sharma, M. Kumar, G. Bhatia, Eur. J. Med. Chem. 45 (2010) 501 (https://doi.org/10.1016/j.ejmech.2009.10.036)
A. Ahamed, I. A. Arif, M. Mateen, R. Surendra Kumar, A. Idhayadhulla, Saudi J. Biol. Sci. 25 (2018) 1227 (https://doi.org/10.1016/j.sjbs.2018.03.001)
A. Gallardo-Godoy, J. Gever, K. L. Fife, B. M. Silber, S. B. Prusiner, A. R. Renslo, J. Med. Chem. 54 (2011) 1010 (https://doi.org/10.1021/jm101250y)
J. V. Urošević, S. Ž. Drmanić , J. B. Nikolić, I. O. Juranić, B. Ž. Jovanović, J. Serb. Chem. Soc. 78 (2013) 1963 (https://doi.org/10.2298/JSC131120139U)
V. Sivamurugan, R. Suresh Kumar, M. Palanichamy, V. Murugesan, J. Heterocycl. Chem. 42 (2005) 743 (https://doi.org/10.1002/jhet.5570420534)
H. N. De Armas, N. Blaton, O. M. Peeters, C. De Ranter, M. Suárez, E. Rolando, Y. Verdecia, E. Ochoa, N. Martín, M. Quinteiro, C. Seoane, J. L. Soto, J. Heterocycl. Chem. 37 (2000) 1575 (https://doi.org/10.1002/jhet.5570370627)
B. Palakshi Reddy, K. Rajesh, V. Vijayakumar, Arab. J. Chem. 8 (2015) 138 (https://doi.org/10.1016/j.arabjc.2011.01.027)
D. B. Shinde, N. D. Shinde, M. S. Shingare, M. P. Dubey, G. K. Patnaik, Indian J. Chem., B 34 (1995) 920
J. Ramchander, Gajula Raju, N. Rameshwar, T. Sheshashena Reddy, A. Ram Reddy, Spectrochim. Acta, A 85 (2012) 210 (https://doi.org/10.1016/j.saa.2011.09.062)
S. Kedare, R. Singh, JFST 48 (2011) 412 (https://doi.org/10.1007/s13197-011-0251-1)
R. Walker, J. D. Everette, J. Agric. Food Chem. 57 (2009) 1156 (https://doi.org/10.1021/jf8026765)
A. Espinel-Ingroff, A. Fothergill, M. Ghannoum, E. Manavathu, L. Ostrosky-Zeichner, M. Pfaller, M. Rinaldi, W. Schell, T. Walsh, J. Clin. Microbiol. 43 (2005) 5243 (https://doi.org/10.1128/JCM.43.10.5243-5246.2005).