Chemical characterization and antimicrobial activity of Juglans nigra L. nut and green husk Scientific paper
Main Article Content
Abstract
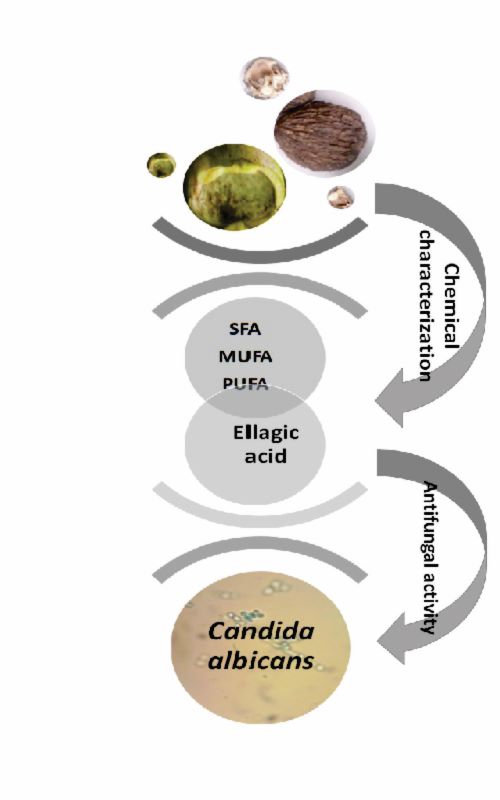
Juglans nigra (Black walnut) is a source of health-supporting biologically active compounds used in traditional medicine. The investigation of bioactive compounds in black walnut could lead to its broader application, as well as to the application of its by-products. Therefore, this study aimed to characterize J. nigra nut and green husk based on chemical analysis of their petroleum ether and ethanol extracts obtained by ultrasonic and reflux extraction methods, respectively. Different extract fractions were tested for their antimicrobial activities using Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa), Gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus) and yeast (reference strain and clinical isolates of Candida albicans). The ethanol extracts analysis, performed by high performance liquid chromatography, singled out the ellagic acid as the most dominant compound in nut ((55.0±1.3)×10-3 kg m-3) and green husk ((114.1±0.5)×10-3 kg m-3) extracts. Non-polar compounds were evaluated using gas chromatography analysis of petroleum ether extracts. Juglans nigra nut and green husk contained two saturated fatty acids, palmitic acid (C16:0) and stearic acid (C18:0), then, monounsaturated fatty acids, palmitoleic (C16:1n-7), oleic (C18:1n-9) and vaccenic acid (C18:1n-7), as well as polyunsaturated fatty acids, linoleic (C18:2n-6), γ-linolenic (C18:3n-6) and α-linolenic (C18:3n-3) acids. Ethanol extracts of both J. nigra nut and green husk showed antimicrobial activity against C. albicans, which is the most common cause of yeast infections.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 0702301;451-03-47/2023-01/200015 -
Science Fund of the Republic of Serbia
Grant numbers 7754282
References
R. Amarowicz, G. A. Dykes, R. B. Pegg, Fitoterapia 79 (2008) 217 (https://doi.org/10.1016/j.fitote.2007.11.019)
K. M. Rajković, M. Vasić, M. Drobac, J. Mutić, S. Jeremić, V. Simić, J. Stanković, Chem. Eng. Res. Des. 157 (2020) 25 (https://doi.org/10.1016/j.cherd.2020.03.002)
J. M. Rorabaugh, A. P. Singh, I. M. Sherrell, M. R. Freeman, N. Vorsa, P. Fitschen, C. Malone, M. A. Maher, T. Wilson, Food Nutr. Sci. 2 (2011) 193 (https://doi.org/10.4236/fns.2011.23026)
J. Wenzel, S. C. Samaniego, L. Wang, L. Burrows, E. Tucker, N. Dwarshuis, M. Ammerman, A. Zand, Food Sci. Nutr. 5 (2017) 223 (https://doi.org/10.1002/fsn3.385)
M. Mirjalili, L. Karimi, J. Chem. (2013) 375352 (http://dx.doi.org/10.1155/2013/375352)
R. S. Camara, V. A. Schlegel, Int. J. Food Prop. 19 (2016) 2175 (https://doi.org/10.1080/10942912.2015.1114951)
K. V. Ho, Z. Lei, L. W. Sumner, M. V. Coggeshall, H. Y. Hsieh, G. C. Stewart, C. H. Lin, Metabolites 29 (2018) 8 (https://doi.org/10.3390/metabo8040058)
K. V. Ho, A. Roy, S. Foote, P. H. Vo, N. Lall, C. H. Lin, Molecules 25 (2020) 4516 (https://doi.org/10.3390/molecules25194516)
D. C. Vu, P. H. Vo, M. V. Coggeshall, C. H. Lin, Food Chem. 66 (2018) 4503 (https://doi.org/10.1021/acs.jafc.8b01181)
D. C. Vu, T. H. D. Nguyenb, T. L. Hoc, RSC Adv. 10 (2020) 33378 (https://doi.org/10.1039/D0RA05714B)
J. N. Ndukauba, I. A. Olawuni, D. C. Okafor, R. O. Enwereuzoh, M. Ojukwu, Int. J. Life Sci. 4 (2015) 58 (https://doi.org/10.13140/RG.2.2.27483.67360)
S. Cosmulescu, I. Trandafir, G. Achim, M. Botu, A. Baciu, M. Gruia, Hort. Agrobot. Cluj 38 (2010) 53 (https://doi.org/10.15835/nbha3814624)
A. Jahanban-Esfahlan, A. Ostadrahimi, M. Tabibiazar, R. A. Amarowicz, Int. J. Mol. Sci. 20 (2019) 3920 (https://doi.org/10.3390/ijms20163920)
A. Salejda, U. Janiewicz, M. Korzeniowska, J. Kolniak-Ostek, K. Grazyna,
LWT-Food Sci. Tech. 65 (2016) 751 (https://doi.org/10.1016/j.lwt.2015.08.069)
B. Shi, W. Zhang, X. Li, X. Pan, Int. J. Food Prop. 20 (2018) 1094 (https://doi.org/10.1080/10942912.2017.1381706)
C. Glaser, H. Demmelmair, B. Koletzko J. Lipid. Res. 51 (2010) 216 (https://doi.org/10.1194/jlr.D000547)
M. Perić, M. Radunović, M. Pekmezović, J. Marinković, R. Živković V. Arsić Arsenijević, J. Prosthodont. 28 (2017) 580 (https://doi.org/10.1111/jopr.12610)
J. L .Rodriquez-Tudela, J. P. Donnelly, M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. Denning, W. Fegeler, P. Gaustad, C. Lass-Flörl, C. Moore, M. Richardson, A. Schmalreck, A. Velegraki, P. Verweij, Clin. Microbiol. Infect. 14 (2008) 982 (https://doi.org/10.1111/j.1469-0691.2008.02086.x)
D. A. Vattem, K. Shetty, J. Food Biochem. 29 (2005) 234 (https://doi.org/10.1111/j.1745-4514.2005.00031.x)
A. D. G. Sampaio, A. V. L. Gontijo, H. M. Araujo, C. Y. Koga-Ito, Antimicrob. Agents Chemother. 26 (2018) 62 (https://doi.org/10.1128/AAC.01716-18)
S. Alfei, B. Marengo G. Zuccari, Antioxidants 9 (2020) 707 (https://doi.org/10.3390/antiox9080707)
J. L. Ríos, R. M. Giner, M. Marín, M. A. Carmen Recio, Planta Medica 84 (2018) 1068 (https://doi.org/10.1055/a-0633-9492)
Z. Papoutsi, E. Kassi, I. Chinou, M. Halabalaki, L. A. Skaltsounis, P. Moutsatsou, Brit. J. Nutr. 99 (2008) 715 (https://doi.org/10.1017/S0007114507837421)
A. Arsic, M. Takic, M. Kojadinovic, S. Petrovic, M. Paunovic, V. Vucic, D. Ristic Medic, Can. J. Physiol. Pharmacol. 99 (2021) 64 (https://doi.org/10.1139/cjpp-2020-0317)
A. Arsić, A. Stojanović, M. Mikić, Ser. J. Exp. Clin. Res. 20 (2019) 3 (https://doi.org/10.1515/sjecr-2017-0077)
D. Ristic-Medic, M. Kovacic, M. Takic, A. Arsic, S. Petrovic, M. Paunovic, V. Vucic, Nutrients 13 (2021) 15 (https://doi.org/10.3390/nu13010015)
A. Arsic, in The Mediterranean Diet, An Evidence-based Approach, V. R. Preedy, R. R. Watson, Eds., Academic Press, An Imprint Elsevier, 2020, pp. 267–274, ISBN: 9780128195789, 2020 (https://doi.org/10.1016/B978-0-12-818649-7.00024-2)
V. Vucic, J. Tepsic, A. Arsic, T. Popovic, J. Debeljak-Martacic, M. Glibetic, Acta Alimentaria 41 (2012) 343 (https://doi.org/10.1556/AAlim.41.2012.3.6)
S.M. Milic, D.M. Kostic, S.P. Milić, M.V. Vučić, C.A. Arsić, B.V. Veljković, O.S. Stamenković, Chem. Eng. Technol. 43 (2020) 1 (https://doi.org/10.1002/ceat.201900689)
A. Naseri, A. Fata, S. A. A. Shamsian, Int. J. Med. Res. Health Sci. 5 (2016) 72 (https://www.ijmrhs.com/medical-research/in-vitro-anticandidal-effects-of-aqueous-and-methanolic-extracts-of-walnut-juglansregia-tree-fruit-peel-in-comparision-w.pdf)
Е. Noumi, М. Snoussi, H. Hajlaoui, E. Valentin, A. Bakhrouf, Eur. J. Clin. Microbiol. Infect. Dis. 29 (2010) 81 (https://doi.org/10.1007/s10096-009-0824-3)
A. D. G. Sampaio, A. V. L. Gontijo, H. M. Araujo, C. Y. Koga-Ito, Agents Chemother. 26 (2018) 62:e01716-18 (https://doi.org/10.1128/AAC.01716-18)
Z. J. Li, X. G. G. Dawuti, S. Aibai, Phytother. Res. 29 (2015) 1019 (https://doi.org/10.1002/ptr.5340)
I. S. Mysiakina, N. S. Funtikova, Mikrobiology 76 (2007) 5 (https://doi.org/10.1134/S0026261707010018).