Star-shaped poly(ε-caprolactones) with well-defined architecture as potential drug carriers Scientific paper
Main Article Content
Abstract
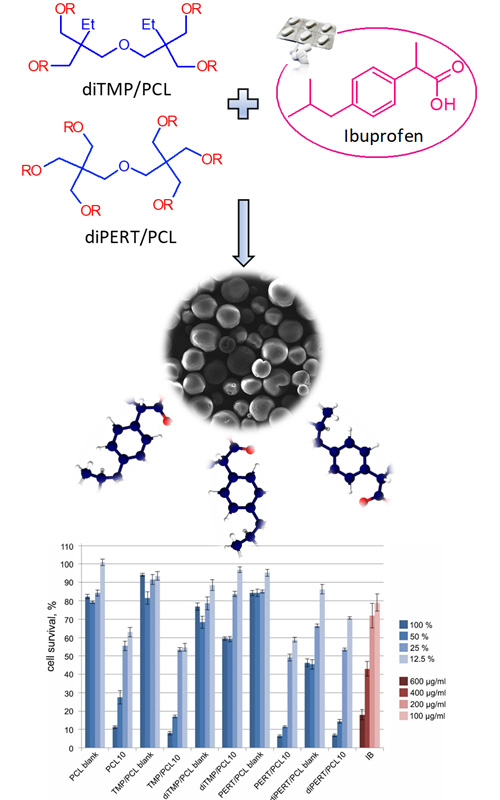
The present study reports the potential application of star-shaped poly(ε-caprolactones) with different number of arms as new drug delivery matrix. Linear and star-shaped PCL ibuprofen loaded microspheres were prepared using oil-in-water (o/w) solvent evaporation technique and characterized with FTIR, DSC, XRD and SEM analysis. High yield, encapsulation efficiency and drug loadings were obtained for all microspheres. FTIR analysis revealed the existence of interactions between polymer matrix and drug, while the DSC analysis suggested that drug was encapsulated in an amorphous form. SEM analysis confirmed that regular, spherical in shape star-shaped microspheres, with diameter between 80 and 90 μm, were obtained, while quite larger microspheres, 110 μm, were prepared from linear PCL. The advantage of using star-shaped PCL microspheres instead of linear PCL was seen from drug release profiles which demonstrated higher amount of drug released from star-shaped polymer matrix as a consequence of their branched, flexible structure. Microspheres prepared from the polymers with the most branched structure showed the highest amount of the released drug after 24 h. Finally, cytotoxicity tests, performed using normal human fibroblasts (MRC5), indicated the absence of cytotoxicity at lower concentrations of microspheres proving the great potential of star-shaped PCL systems in comparison to linear ones.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200135
References
A. K. Bajpai, S. K. Shukla, S. Bhanu, S. Kankane, Prog. Polym. Sci. 33 (2008) 1088 (http://dx.doi.org/10.1016/j.progpolymsci.2008.07.005)
W. Wu, W. Wang, J. Li, Prog. Polym. Sci. 46 (2015) 55 (https://doi.org/10.1016/j.progpolymsci.2015.02.002)
J. M. Ren, T. G. McKenzie, Q. Fu, E. H. H. Wong, J. Xu, Z. An, S. Shanmugam, T. P. Davis, C. Boyer, G. G. Qiao, Chem. Rev. 116 (2016) 6743 (https://doi.org/10.1021/acs.chemrev.6b00008)
C. Englert, J. C. Brendel, T. C. Majdanski, T. Yildirim, S. Schubert, M. Gottschaldt, N. Windhab, U. S. Schubert, Prog. Polym. Sci. 87 (2018) 107 (https://doi.org/10.1016/j.progpolymsci.2018.07.005)
D. J. A. Cameron, M. P. Shaver, Chem. Soc. Rev. 40 (2011) 1761 (https://doi.org/10.1039/c0cs00091d)
K. Khanna, S. Varshney, A. Kakkar, Polym. Chem.-U.K. 1 (2010) 1171 (https://doi.org/10.1039/C0PY00082E)
M. Hadjianfar, D. Semnani, J. Varshosaz, Polym. Advan. Technol. 29 (2018) 2972 (https://doi.org/10.1002/pat.4417)
M. A. Woodruff, D. W. Hutmacher, Prog. Polym. Sci. 35 (2010) 1217 (https://doi.org/10.1016/j.progpolymsci.2010.04.002)
J. L. Wang, L. Wang, C. M. Dong, J. Polym. Sci. Pol. Chem. 43 (2005) 5449 (https://doi.org/10.1002/pola.20954)
A. Michalski, M. Brzezinski, G. Lapienis, T. Biela, Prog. Polym. Sci. 89 (2019) 159 (https://doi.org/10.1016/j.progpolymsci.2018.10.004)
M. Lang, R. P. Wong, C. C. Chu, J. Polym. Sci. Pol. Chem. 40 (2002) 1127 (https://doi.org/10.1002/pola.10171)
M. R. Nabid, S. J. Tabatabaei Rezaei, R. Sedghi, H. Niknejad, A. A. Entezami, H. A. Oskooie, M. M. Heravi, Polymer 52 (2011) 2799 (https://doi.org/10.1016/j.polymer.2011.04.054)
H. J. Lim, H. Lee, K. H. Kim, J. Huh, C. H. Ahn, J. W. Kim, Colloid. Polym. Sci. 291 (2013) 1817 (https://doi.org/10.1007/s00396-013-2916-y)
S. Petrova, S. Miloshev, M. D. Apostolova, R. Mateva, J. Mater. Sci.-Mater. Med. 23 (2012) 1225 (https://doi.org/10.1007/s10856-012-4592-8)
F. Quaglia, L. Ostacolo, G. De Rosa, M. I. La Rotonda, M. Ammendola, G. Nese, G. Maglio, R. Palumbo, C. Vauthier, Int. J. Pharmaceut. 324 (2006) 56 (https://doi.org/10.1016/j.ijpharm.2006.07.020)
K. S. Shalaby, M. E. Soliman, G. Bonacucina, M. Cespi, G. F. Palmieri, O. A. Sammour, A. A. El Shamy, L. Illum, L. Casettari, Pharm. Res.-DORDR 33 (2016) 2010 (https://doi.org/10.1007/s11095-016-1939-8)
C. D. Brabander, C. Vervaet, L. V. Bortel, J. P. Remon, Int. J. Pharmaceut. 271 (2004) 77 (https://doi.org/10.1016/j.ijpharm.2003.10.029)
N. Carreras, V. Acuña, M. Martí, M. J. Lis, Colloid. Polym. Sci. 291 (2013) 157 (https://doi.org/10.1007/s00396-012-2768-x)
G. V. Salmoria, F. Sibilia, I. M. Gindri, C. R. M. Roesler, S. Farè, M. C. Tanzi, Polym. Test. 62 (2017) 33 (https://doi.org/10.1016/j.polymertesting.2017.06.009)
T. Shpigel, G. Cohen Taguri, D. Y. Lewitus, J. Appl. Polym. Sci. 136 (2019) 47227 (https://doi.org/10.1002/app.47227)
M. Ponjavic, M. S. Nikolic, J. Nikodinovic-Runic, T. Ilic-Tomic, J. Djonlagic, Int. J. Polym. Mater. PO 68 (2019) 308 (https://doi.org/10.1080/00914037.2018.1445631)
M. Ponjavic, M. S. Nikolic, S. Stevanovic, J. Nikodinovic-Runic, S. Jeremic, A. Pavic, J. Djonlagic, J. Bioact. Compat. Pol. 35 (2020) 517 (https://doi.org/10.1177/0883911520951826)
D. Pepic, M. S. Nikolic, S. Grujic, M. Lausevic, J. Djonlagic, J. Microencapsul. 30 (2013) 151 (https://doi.org/10.3109/02652048.2012.704954)
M. B. Hansen, S.E. Nielsen, K. Berg, J. Immunol. Methods 119 (1989) 203 (https://doi.org/10.1016/0022-17598990397-9)
M. Jaiswal, V. Koul, J. Biomater. Appl. 27 (2013) 848 (https://doi.org/10.1177/0885328211428524)
Y. Jiang, K. Mao, X. Cai, S. Lai, X. Chen, J. Appl. Polym. Sci. 122 (2011) 2309 (https://doi.org/10.1002/app.34382)
K. J. Zhu, Y. Li, H. L. Jiang, H. Yasuda, A. Ichimaru, K. Yamamoto, P. Lecomte, R. Jerome, J. Microencapsul. 22 (2005) 25 (https://doi.org/10.1080/02652040400026350)
D. Bikiaris, G. Z. Papageorgiou, A. Stergiou, E. Pavlidou, E. Karavas, F. Kanaze, M. Georgarakis, Thermochim. Acta 439 (2005) 58 (https://doi.org/10.1016/j.tca.2005.09.011)
J. Bidone, A. P. P. Melo, G. C. Bazzo, F. Carmignan, M. S. Soldi, A. T. N. Pires, E. Lemos-Senna, Mater. Sci. Eng., C 29 (2009) 588 (https://doi.org/10.1016/j.msec.2008.10.016)
A. Baji, S. C. Wong, T. Liu, T. Li, T. S. Srivatsan, J. Biomed. Mater. Res., B 81 (2007) 343 (https://doi.org/10.1002/jbm.b.30671)
S. Freiberg, X. X. Zhu, Int. J. Pharmaceut. 282 (2004) 1 (https://doi.org/10.1016/j.ijpharm.2004.04.013)
C. Koulouktsi, S. Nanaki, P. Barmpalexis, M. Kostoglou, D. Bikiaris, Int. J. Pharmaceut. 1 (2019) 100014 (https://doi.org/10.1016/j.ijpx.2019.100014)
P. Costa, J. M. Sousa Lobo, Eur. J. Pharm. Sci. 13 (2001) 123 (https://doi.org/10.1016/s0928-09870100095-1)
C. J. Thompson, D. Hansford, S. Higgins, C. Rostron, G.A. Hutcheon, D.L. Munday, Int. J.Pharmaceut. 329 (2007) 53 (https://doi.org/10.1016/j.ijpharm.2006.08.019)
M. C. Serrano, R. Pagani, M. Vallet-Regı́, J. Peña, A. Rámila, I. Izquierdo, M. T. Portolés, Biomaterials 25 (2004) 5603 (https://doi.org/10.1016/j.biomaterials.2004.01.037).