Theoretical study on the insertion reaction of the phosphenium cation and azirane Scientific paper
Main Article Content
Abstract
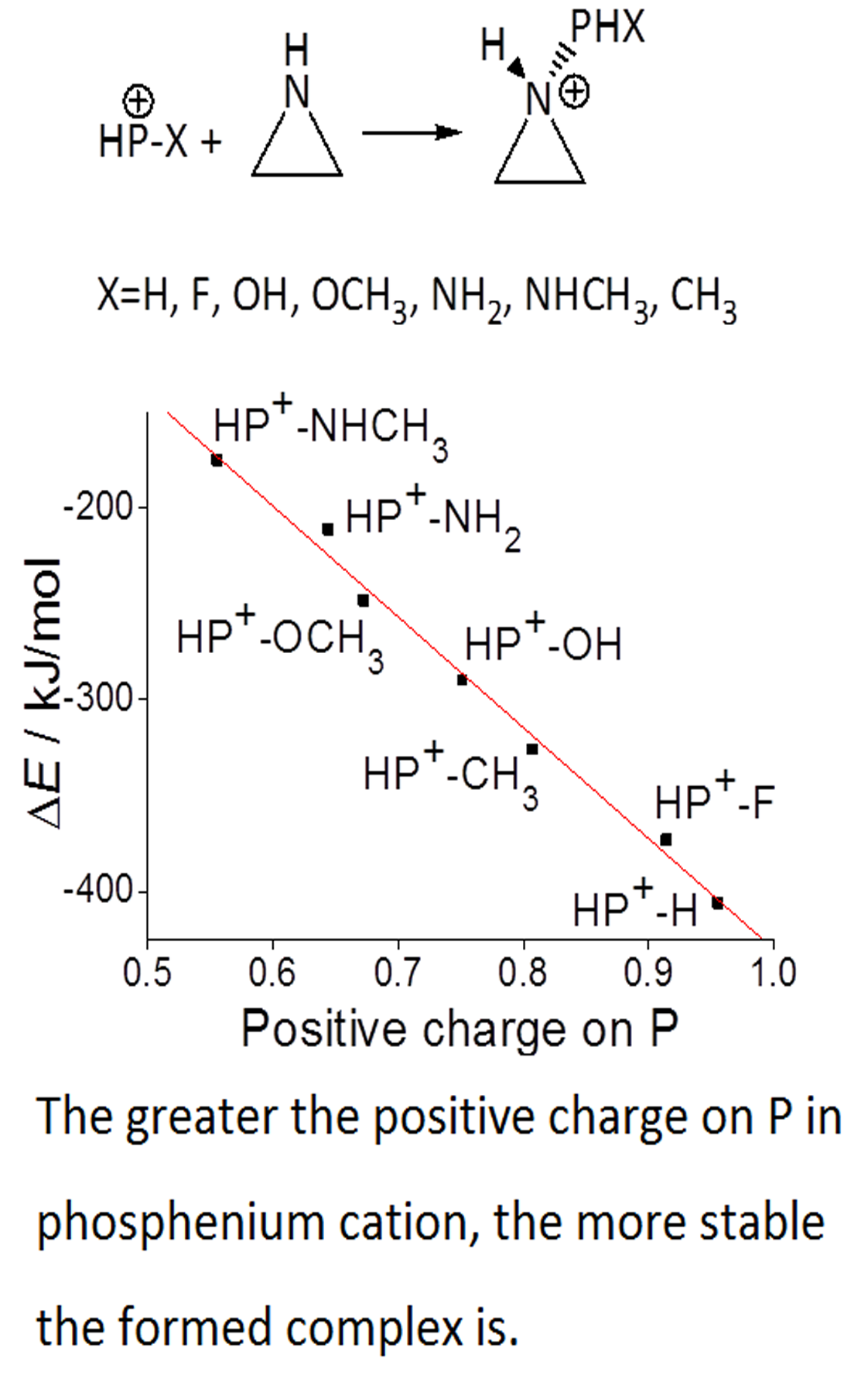
The mechanism of the insertion reaction between the phosphenium cation and azirane has been investigated theoretically in order to better understand the reactivity for the valence isoelectronic of carbene. The phosphenium cation acts as an electrophilic reagent and accepts the σ electrons of azirane to form a complex in the first combination step. The greater the positive charge on the phosphorus in the phosphenium cation, the more stable is the formed complex. Introduction of substituents will decrease the positive charge on the phosphorus in the phosphenium cation. The order of positive charge on phosphorus is HP+–F > HP+–OH > HP+–NH2, which is consistent with their Lewis acidities. The complex transforms to a four-membered ring product via a transition state in the second insertion step. The product is more stable than the complex due to the decrease of the ring extension.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Natural Science Foundation of Shandong Province
Grant numbers ZR2020MC004 -
Science and Technology Development Plan of Shandong Province
Grant numbers 2019JZZY011116
References
J. F. Harrison, R. C. Liedtke, J. F. Liebman, J. Am. Chem. Soc. 101 (1979) 162 (https://doi.org/10.1021/ja00518a006)
D. E. Falvey, C. J. Cramer, Tetrahedron Lett. 33 (1992) 1705 (https://doi.org/10.1016/S0040-4039(00)91711-8)
J. Berkowitz, L. A. Curtiss, S. T. Gibson, J. P. Greene, J. Chem. Phys. 84 (1986) 375 (https://doi.org/10.1063/1.450147)
J. F. Harrison, J. Am. Chem. Soc. 103 (1981) 7406 (https://doi.org/10.1021/ja00415a002)
H. M. Cheng, C. F. Lin, S. Y. Chu, J. Phys. Chem., A 111 (2007) 6890 (https://doi.org/10.1021/jp070812i)
M. G. Thomas, C. W. Schultz, R. W. Parry, Inorg. Chem. 16 (1977) 994 (https://doi.org/10.1021/ic50171a005)
J. C. Clyburne, M. J. Schriver, Inorg. Chem. 35 (1996) 3062 (https://doi.org/10.1021/ic951132+)
G. David, E. Niecke, M. Nieger, J. Radseck, W. W. Schoeller, J. Am. Chem. Soc. 116 (1994) 2191 (https://doi.org/10.1021/ja00084a088)
J. W. Larson, T. B. McMahon, Inorg. Chem. 26 (1987) 4018 (https://doi.org/10.1021/ic00271a011)
J. M. Slattery, S. Hussein, Dalton Trans. 41 (2012) 1808 (https://doi.org/10.1039/c1dt11636c)
H. P. Műller, D. E. Woon, J. Phys. Chem., A 117 (2013) 13868 (https://doi.org/10.1021/jp4083807)
C. A. Dyker, N. Burford, Chem. Asian J. 3 (2008) 28 (https://doi.org/10.1002/asia.200700229)
N. Burford, P. J. Ragogna, R. McDonald, M. J. Ferguson, J. Am. Chem. Soc. 125 (2003) 14404 (https://doi.org/10.1021/ja036649w)
J. J.Weigand, M. Holthausen, R. Fröhlich, Angew. Chem. Int. Ed. 48 (2009) 295 (https://doi.org/10.1002/anie.200804903)
N. E. Brasch, I. G. Hamilton, E. H. Krenske, S. Bruce, Organometallics 23 (2004) 299 (https://doi.org/10.1021/om030607z)
B. Breit, J. Mol. Catal., A 143 (1999) 143 (https://doi.org/10.1016/S1381-1169(98)00377-X)
A. H. Cowley, R. A. Kemp, Chem. Rev. 85 (1985) 367 (https://doi.org/10.1021/cr00069a002)
T. Krachko, J. C. Slootweg, Eur. J. Inorg. Chem. 24 (2018) 2734 (https://doi.org/10.1002/ejic.201800459)
M. B. Abrams, B. L. Scott, R. T. Baker, Organometallics 19 (2000) 4944 (https://doi.org/10.1021/om0005351)
D. Gudat, A. Haghverdi, M. Nieger, J. Organomet. Chem. 617 (2001) 383 (https://doi.org/10.1016/S0022-328X(00)00624-0)
S. Burck, D. Gudat, Inorg. Chem. 47 (2008) 315 (https://doi.org/10.1021/ic7017049)
S. G. He, B. S. Tackett, D. J. Clouthier, J. Chem. Phys. 121 (2004) 257 (https://doi.org/10.1063/1.1758699)
C. Lee, W. Yang, R.G. Parr, Phys. Rev., B 37 (1988) 785 (https://doi.org/10.1103/PhysRevB.37.785)
Y. Zhao, W. H. Wang, W. L. Feng, W. L. Wang, P. Li, J. Phys. Chem., A 122 (2018) 7312 (https://doi.org/10.1021/acs.jpca.8b04775)
K. Xu, W. W. Wang, W. J. Wei, W. L. Feng, Q. Sun, P. Li, J. Phys. Chem., A 121 (2017) 7236 (https://doi.org/10.1021/acs.jpca.7b05858)
Gaussian 09, Gaussian, Inc., Wallingford, CT, 2010 (https://gaussian.com/g09citation/).