Kinetic investigation of reactions of a 3-arylidene-2-thiohydantoin derivative with palladium(II) salts Scientific paper
Main Article Content
Abstract
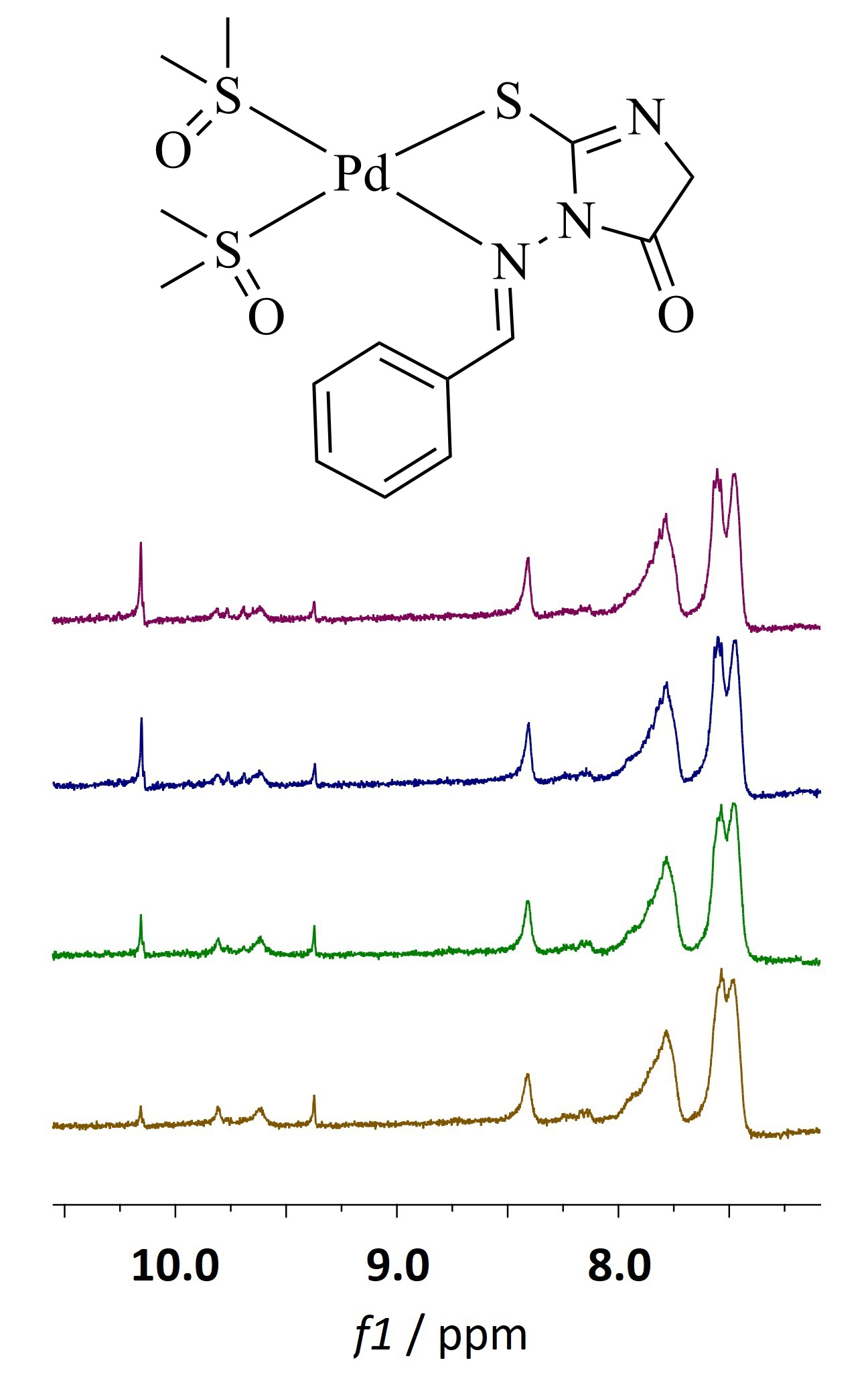
1H-NMR spectroscopy was used to monitor the reactions of an arylidene 2-thiohydantoin derivative, 3-((phenylmethylene)amino)-2-thioxo-4-imidazolidinone (3), with PdCl2, cis-[PdCl2(dmso-S)2] and K2[PdCl4] in DMSO-d6 in order to elucidate the reaction kinetics and mechanism. The 2-thiohydantoin derivative 3 formed cis-[Pd(3-N,S)(dmso-S)2]+ complex (5) in reactions with PdCl2 and cis-[PdCl2(dmso-S)2], while no reaction with K2[PdCl4] was observed.
A two-step mechanism for the reactions of 3 with PdCl2 and cis-[PdCl2(dmso-S)2] is proposed, in which fast coordination to the side chain nitrogen occurs in the first step, while chelation and coordination to the sulfur atom in the 2-thiohydantoin ring is the second, slower, rate-determining step. The reaction rate constants were calculated and reactivities of the 2-thiohydantoin derivative 3 towards the palladium(II) salts were compared and discussed. Reaction of 3 with cis-[PdCl2(dmso-S)2] was faster than with PdCl2. The investigated palladium(II) salts also react with the solvent, DMSO-d6, and the influence of these side reactions on the outcome and kinetics of the 2-thiohydantoin derivative complexation reaction is discussed in detail. The obtained results of this study can have an impact in explanation of the coordination behavior of antitumor active palladium(II) and platinum(II) complexes.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Fakultet Medicinskih Nauka, Univerziteta U Kragujevcu
Grant numbers JP02/20 -
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200378, 451-03-68/2022-14/200252 and 451-03-47/2023-01/200111
References
M. A. Metwally, E. Abdel-Latif, J. Sulfur Chem. 33 (2012) 229 (https://www.doi.org/10.1080/17415993.2011.643550)
S. H. Cho, S. H. Kim, D. Shin, Eur. J. Med. Chem. 164 (2019) 517 (https://doi.org/10.1016/j.ejmech.2018.12.066)
P. P. Gawas, B. Ramakrishna, N. Veeraiah, V. Nutalapati, J. Mater. Chem., C 9 (2021) 16341 (https://www.doi.org/10.1039/D1TC04090A)
J. Marton, J. Enisz, S. Hosztafi, T. Timar, J. Agr. Food Chem. 41 (1993) 148 (https://www.doi.org/10.1021/jf00025a031)
A. I. Khodair, H. I. el-Subbagh, A. A. el-Emam, Boll. Chim. Farm. 136 (1997) 561 (https://pubmed.ncbi.nlm.nih.gov/9440349)
A. M. Al-Obaid, H. I. El-Subbagh, A. Khodair, M. M. A. Elmazar, Anti-cancer Drug 7 (1996) 873 (https://www.doi.org/10.1097/00001813-199611000-00009)
S. Suzen, E. Buyukbingol, Farmaco 55 (2000) 246 (https://www.doi.org/10.1016/S0014-827X(00)00028-8)
A. C. W. Curran, U.S. Patent 3,984,430 (1976)
M. M. W. Habib, M. A. O. Abdelfattah, A. H. Abadi, Arch. Pharm. 348 (2015) 868 (https://www.doi.org/10.1002/ardp.201500272)
A. Takahashi, H. Matsuoka, Y. Uda, Environ. Mutagen Res. 26 (2004) 1 (https://www.doi.org/10.3123/jems.26.1)
H. R. Kim, H. J. Lee, Y. J. Choi, Y. J. Park, Y. Woo, S. J. Kim, M. H. Park, H. W. Lee, P. Chun, H. Y. Chung, H. R. Moon, Med. Chem. Commun. 5 (2014) 1410 (https://www.doi.org/10.1039/C4MD00171K)
B. Mo, J. Li, S. Liang, Anal. Biochem. 252 (1997) 169 (https://www.doi.org/10.1006/abio.1997.2278)
J. Nelson, M. Helber, M. Brick, U.S. Patent 5,695,917 (1997)
S. S. Kandil, G. B. El-Hefnawy, E. A. Baker, Thermochim. Acta 414 (2004) 105 (https://www.doi.org/10.1016/j.tca.2003.11.021)
J. A. Crim, H. G. Petering, Cancer Res. 27 (1967) 1278 (https://pubmed.ncbi.nlm.nih.gov/4952520)
V. R. Martínez, M. V. Aguirre, J. S. Todaro, E. G. Ferrer, P. A. M. Williams, Biol. Trace Elem. Res. 197 (2020) 454 (https://www.doi.org/10.1007/s12011-019-02013-w)
M. Pitucha, A. Korga-Plewko, A. Czylkowska, B. Rogalewicz, M. Drozd, M. Iwan, J. Kubik, E. Humeniuk, G. Adamczuk, Z. Karczmarzyk, E. Fornal, W. Wysocki, P. Bartnik, Int. J. Mol. Sci. 22 (2021) 3104 (https://www.doi.org/10.3390/ijms22063104)
R. M. El-Bahnasawy, M. M. Shoukry, M. M. Hussein, J. Chem. Sci. 96 (1986) 309 (https://www.doi.org/10.1007/BF02895726)
D. C. Dash, F. M. Meher, P. C. Mohanty, J. Nanda, Indian J. Chem., A 26 (1987) 698 (http://nopr.niscpr.res.in/handle/123456789/47907)
S. Abdullah, R. Al Hassani, A. J. Atia, A. Hussein, Acta Chim. Pharm. Indica 6 (2016) 80 (https://www.tsijournals.com/abstract/synthesis-characterization-and-enzyme-activity-of-coii-niii-cuii-pdii-ptiv-and-cdii-complexes-with-2thioxoimidazolidin4o-11471.html)
K. Tishchenko, E. Beloglazkina, M. Proskurnin, V. Malinnikov, D. Guk, M. Muratova, O. Krasnovskaya, A. Udina, D. Skvortsov, R. R. Shafikov, Y. Ivanenkov, V. Aladinskiy, I. Sorokin, O. Gromov, A. Majouga, N. Zyk, J. Inorg. Biochem. 175 (2017) 190 (https://www.doi.org/10.1016/j.jinorgbio.2017.07.015)
A. Fedorchuk, E. Goreshnik, Y. Slyvka, M. Mys’kiv, Acta Chim. Slov. 67 (2020) 1148 (https://www.doi.org/10.17344/acsi.2020.6045)
P. Arrizabalage, P. Castan, J.-P. Laurent, Transit. Met. Chem. 5 (1980) 324 (https://www.doi.org/10.17344/acsi.2020.6045)
J. S. Casas, E. E. Castellano, M. D. Couce, N. Playá, A. Sánchez, J. Sordo, J. M. Varela, J. Zukerman-Schpector, J. Coord. Chem. 47 (1999) 299 (https://www.doi.org/10.1080/00958979908023062)
R. M. Mahfouz, A. S. El Shahawy, A. A. Hassan, Transit. Met. Chem. 19 (1994) 385 (https://www.doi.org/10.1007/BF00139309)
D. C. Dash, P. Naik, S. K. Naik, R. K. Mohapatra, S. Ghosh, J. Indian Chem. Soc. 86 (2009) 969 (https://doi.org/10.5281/zenodo.5816598)
L. A. Ismail, R. Zakaria, E. M. Hassan, M. Y. Alfaifi, A. A. Shati, S. E. I. Elbehairi, A. A. El-Bindary, R. F. M. Elshaarawy, RSC Adv. 12 (2022) 28364 (https://www.doi.org/10.1039/D2RA05233D)
B. Šmit, R. Z. Pavlović, A. Radosavljević-Mihailović, A. Došen, M. G. Ćurčić, D. S. Šeklić, M. N. Živanović, J. Serb. Chem. Soc. 78 (2013) 217 (https://www.doi.org/10.2298/JSC120725154S)
P. E. Allegretti, M. de las Mercedes Schiavoni, C. Guzmán, A. Ponzinibbio, J. J. P. Furlong, Eur. J. Mass Spectrom. 13 (2007) 291 (https://www.doi.org/10.1255/ejms.885)
B. F. G. Johnson, J. Puga, P. R. Raithby, Acta Crystallogr., B 37 (1981) 953 (https://www.doi.org/10.1107/S0567740881004743)
B. B. Wayland, R. F. Schramm, Inorg. Chem. 8 (1969) 971 (https://www.doi.org/10.1021/ic50074a050)
J. Selbin, W. E. Bull, L. H. Holmes, J. Inorg. Nucl. Chem. 16 (1961) 219 (https://www.doi.org/10.1016/0022-1902(61)80493-4)
L. I. Elding, A. B. Gröning, Inorg. Chim. Acta 31 (1978) 243 (https://www.doi.org/10.1016/s0020-1693(00)95010-2).