Structure and DNA/BSA binding study of zinc(II) complex with 4-ethynyl-2,2’-bipyridine Scientific paper
Main Article Content
Abstract
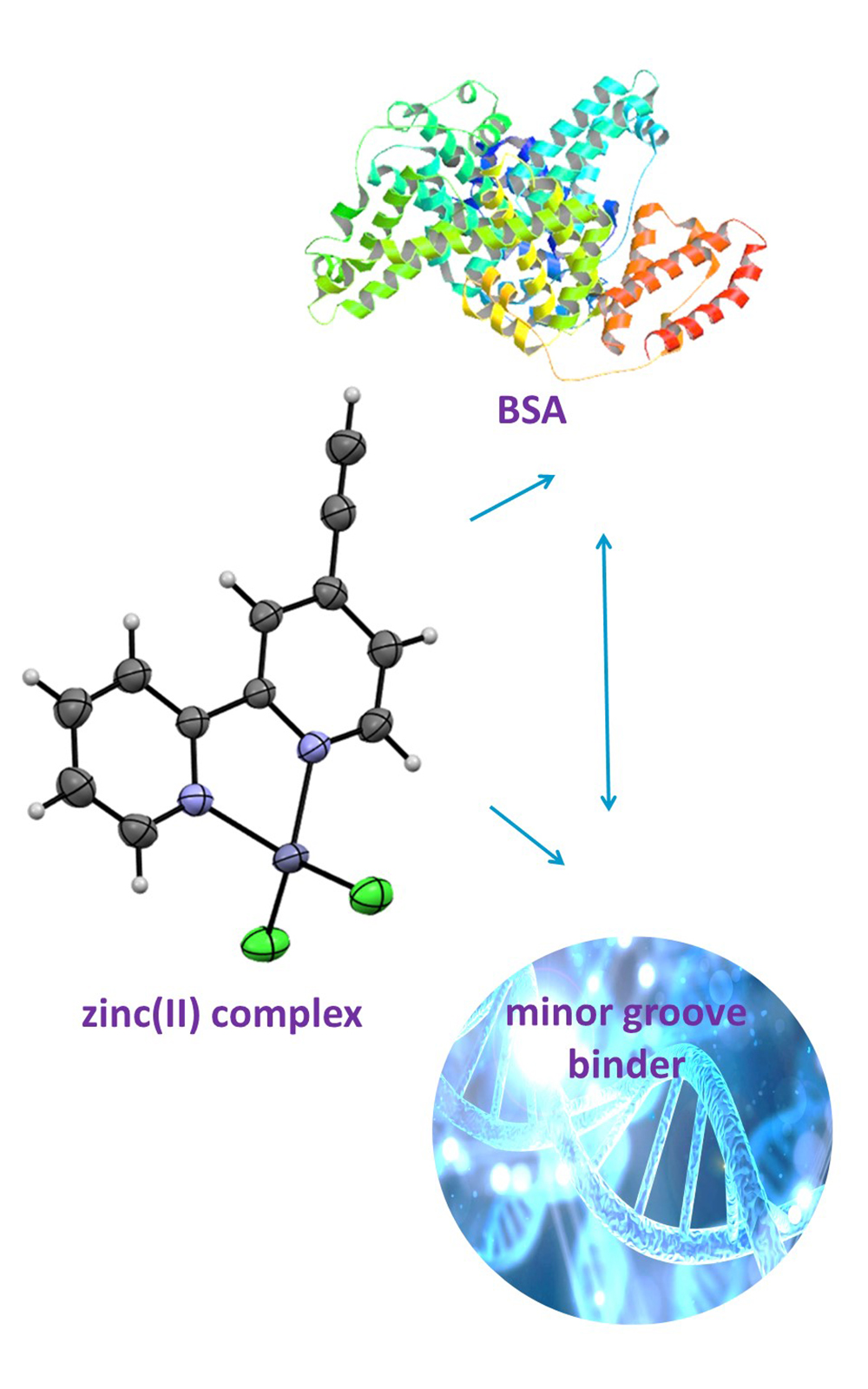
In the present study, a zinc(II) complex with 4-ethynyl-2,2’-bipyridine (ebpy), [Zn(ebpy)Cl2], was synthesized and characterized by spectroscopic (1H-NMR, IR and UV–Vis) methods and molar conductivity measurement. The crystal structure of the [Zn(ebpy)Cl2] complex was determined by single-crystal X-ray diffraction analysis, confirming the bidentate coordination of the ebpy ligand through its two nitrogen atoms, while the remaining two coordination sites are occupied by two chloride ions. With the aim to investigate the reactivity of the synthesized zinc(II) complex toward biologically important molecules, its binding affinity to calf thymus DNA (ct-DNA) and bovine serum albumin (BSA) was studied by fluorescence emission spectroscopy. From the obtained results, it can be concluded that [Zn(ebpy)Cl2] complex binds to bovine serum albumin reversibly, while the combination of ethidium bromide (EthBr) and Hoechst 33258 (2’-(4-hydroxyphenyl)-5-[5-(4-methylpiperazine-1-yl)benzimidazo-2-yl]-benzimidazole) competitive binding study suggests that this complex interacts with ct-DNA through the minor groove binding, which is in agreement with molecular docking study.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-451-03-47/2023-01/200122;451-03-47/2023-01/200378 -
Serbian Academy of Sciences and Arts
Grant numbers F128
References
S. Frassinetti, G. L. Bronzetti, L. Caltavuturo, M. Cini, C. Della Croce, J. Environ. Pathol. Toxicol. Oncol. 25 (2006) 597 (https://dx.doi.org/10.1615/JEnvironPatholToxicolOncol.v25.i3.40 )
J. Burgess, R. H. Prince, in Encyclopedia of Inorganic Chemistry, John Wiley & Sons, Ltd., New York, 2006 (https://dx.doi.org/10.1002/0470862106.ia260 )
J. Osredkar, N. Sustar, J. Clin. Toxicol. S3 (2011) 495 (https://doi.org/0.4172/2161- 0495.S3-001)
B. K. Y. Bitanihirwe, M. G. Cunningham, Synapse 63 (2009) 1029 (https://doi.org/10.1002/syn.20683)
A. S. Prasad, F. W. J. Beck, D. C. Snell, M. Kucuk, Nutr. Cancer 61 (2009) 879 (https://doi.org/10.1080/01635580903285122)
H. Vahrenkamp, Dalton Trans. (2007) 4751 (https://doi.org/10.1039/B712138E)
S. N. Sovari, F. Zobi, Chemistry 2 (2020) 418 (https://doi.org/10.3390/chemistry2020026)
M. Pellei, F. Del Bello, M. Porchia, C. Santini, Coord. Chem. Rev. 445 (2021) 214088 (https://doi.org/10.1016/j.ccr.2021.214088)
Y. Yoshikawa, H. Yasui, Curr. Top. Med. Chem. 12 (2012) 210 (https://doi.org/10.2174/156802612799078874)
G. Psomas, Coord. Chem. Rev. 412 (2020) 213259 (https://doi.org/10.1016/j.ccr.2020.213259)
Q. Zhou, T. W. Hambley, B. J. Kennedy, P. A. Lay, P. Turner, B. Warwick, J. R. Biffin, H. L. Regtop, Inorg. Chem. 39 (2000) 3742 (https://doi.org/10.1016/10.1021/ic991477i)
T. P. Andrejević, I. Aleksic, J. Kljun, B. V. Pantović, D. Milivojevic, S. Vojnovic, I. Turel, M. I. Djuran, B. Ð. Glišić, Inorganics 10 (2022) 71 (https://doi.org/10.3390/inorganics10060071)
J. Rossier, D. Hauser, E. Kottelat, B. Rothen-Rutishauser, F. Zobi, Dalton Trans. 46 (2017) 2159 (https://doi.org/10.1039/C6DT04443C)
J. Rossier, J. Delasoie, L. Haeni, D. Hauser, B. Rothen-Rutishauser, F. Zobi, J. Inorg. Biochem. 209 (2020) 111122 (https://doi.org/10.1016/j.jinorgbio.2020.111122)
N. Zabarska, D. Sorsche, F. W. Heinemann, S. Glump, S. Rau, Eur. J. Inorg. Chem. 2015 (2015) 4869 (https://doi.org/10.1002/ejic.201500630)
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr. 42 (2009) 339 (https://doi.org/10.1107/S0021889808042726)
G. M. Sheldrick, Acta Crystallogr., A 71 (2015) 3 (https://doi.org/10.1107/S2053273314026370)
G. M. Sheldrick, Acta Crystallogr., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
P. Smoleński, C. Pettinari, F. Marchetti, M. F. C. Guedes da Silva, G. Lupidi, G. V. B. Patzmay, D. Petrelli, L. A. Vitali, A. J. L. Pomberio, Inorg. Chem. 54 (2015) 434 (https://doi.org/10.1021/ic501855k)
D. S. Raja, N. S. P. Bhuvanesh, K. Natarajan, Inorg. Chem. 50 (2011) 12852 (https://doi.org/10.1021/ic2020308)
C. A. Puckett, J. K. Barton, J. Am. Chem. Soc. 129 (2007) 46 (https://doi.org/10.1021/ja0677564)
R. Bera, B. K. Sahoo, K. S. Ghosh, S. Dasgupta, Int. J. Biol. Macromol. 42 (2008) 14 (https://doi.org/10.1016/j.ijbiomac.2007.08.010)
K. Schindler, Y. Cortat, M. Nedyalkova, A. Crochet, M. Lattuada, A. Pavic, F. Zobi, Pharmaceuticals 15 (2022) 1107 (https://doi.org/10.3390/ph15091107)
H. R. Drew, R. M. Wing, T. Takano, C. Broka, S. Tanaka, K. Itakura, R. E. Dickerson, Proc. Natl. Acad. Sci. U.S.A. 78 (1981) 2179 (https://doi.org/10.1073/pnas.78.4.2179)
O. Trott, A. J. Olson, J. Comput. Chem. 31 (2010) 455 (https://doi.org/10.1002/jcc.21334)
G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell, A. J. Olson, J. Comput. Chem. 30 (2009) 2785 (https://doi.org/10.1002/jcc.21256)
L. Yang, D.R. Powell, R. P. Houser, Dalton Trans. (2007) 955 (https://doi.org/10.1039/B617136B)
F. H. Allen, Acta Crystallogr.,B 58 (2002) 380 (https://doi.org/10.1107/S0108768102003890)
I. Ali, W. A. Wani, K. Saleem, Synth. React. Inorg. Met.-Org. Chem. 43 (2013) 1162 (https://doi.org/10.1080/15533174.2012.756898)
T. P. Andrejević, B. Warżajtis, B. Đ. Glišić, S. Vojnovic, M. Mojicevic, N. Lj. Stevanović, J. Nikodinovic-Runic, U. Rychlewska, M. I. Djuran, J. Inorg. Biochem. 208 (2020) 111089 (https://doi.org/10.1016/j.jinorgbio.2020.111089)
V. T. Yilmaz, C. Icsel, J. Batur, S. Aydinlik, M. Cengiz, O. Buyukgungor, Dalton Trans. 46 (2017) 8110 (https://doi.org/10.1039/c7dt01286a)
Y. Shi, C. Guo, Y. Sun, Z. Liu, F. Xu, Y. Zhang, Z. Wen, Z. Li, Biomacromolecules 12 (2011) 797 (https://doi.org/10.1021/bm101414w)
O. H. Laitinen, V. P. Hytönen, H. R. Nordlund, M. S. Kulomaa, Cell. Mol. Life Sci. 63 (2006) 2992 (https://doi.org/10.1007/s00018-006-6288-z)
A. K. Ghose, V. N. Viswanadhan, J. J. Wendoloski, J. Comb. Chem. 1 (1999) 55 (https://doi.org/10.1021/cc9800071)
C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug Deliv. Rev. 23 (1997) 3 (https://doi.org/10.1016/S0169-409X(96)00423-1).