Influence of electrochemical conditions on the regio- and stereoselectivity of selenocyclization of alkenyl hydantoins Short Communication
Main Article Content
Abstract
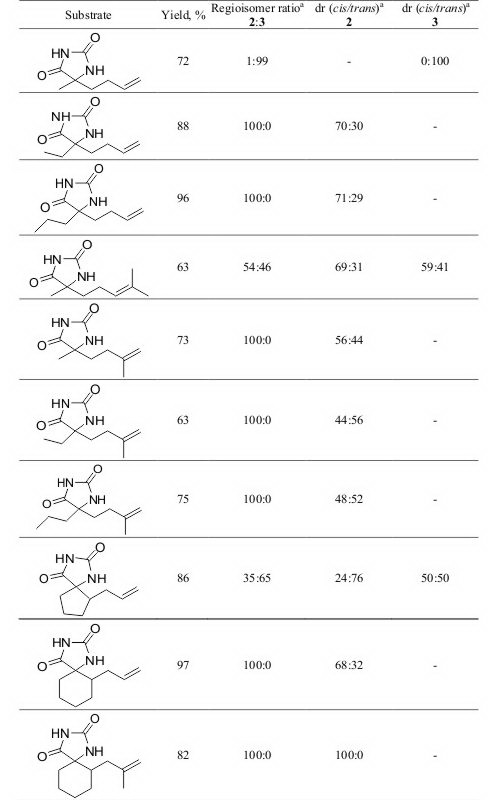
5-Alkenyl hydantoins and alkenyl spirohydantoins are converted into bicyclic and tricyclic hydantoins, under indirect electrochemical conditions, generating the phenylselenyl cation in situ. The reactions proceeded in good to exelent yields. The influence of electrochemical conditions on regio- and diastereoselectivity of the selenocyclization reactions is investigated.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
Y. Jiang, K. Xu, C. Zeng, Chem. Rev. 118 (2018) 4485 (https://doi.org/10.1021/acs.chemrev.7b00271)
J. C. Thenmozhiyal, P.T. Wong, W. Chui, J. Med. Chem. 47 (2004) 1527 (https://dx.doi.org/10.1021/jm030450c)
A. Volonterio, C. R. de Arellano, M. Zanda, J. Org. Chem. 70 (2005) 2161 (https://dx.doi.org/10.1021/jo0480848)
Y. Fujiwara, G. C. Fu, J. Am. Chem. Soc. 133 (2011) 12293 (https://dx.doi.org/10.1021/ja2049012)
F. Brockmeyer, D. Kröger, T. Stalling, P. Ullrich, J. Martens, Helv. Chim. Acta 95 (2012) 1857 (https://dx.doi.org/10.1002/hlca.201200441)
D. W. Knight, Prog. Heterocyc. Chem. 14 (2002) 19 (https://dx.doi.org/10.1016/S0959-6380(02)80004-6)
N. Petragnani, H. A. Stefani, C. J. Valduga, Tetrahedron 57 (2001)1411 (https://dx.doi.org/10.1016/S0040-4020(00)01033-4)
B. M. Šmit, R. Z. Pavlović, Tetrahedron 71 (2015) 1101 (https://dx.doi.org/10.1016/j.tet.2014.12.088)
B. M. Šmit, M. Rodić, R. Z. Pavlović, Synthesis-Stuttgart 48 (2016) 387 (https://dx.doi.org/10.1055/s-0035-1561285)
P. Röse, S. Emge, J. Yoshida, G. Hilt, Beilstein J. Org. Chem. 11 (2015) 174 (https://dx.doi.org/10.3762/bjoc.11.18)
D. Stevanović, A. Pejović, M. D. Vukićević, G. Dobrikov, V. Dimitrov, M. S. Denić, N. S. Radulović, R. D. Vukićević, Helv. Chim. Acta 96 (2013) 1103 (https://dx.doi.org/10.1002/hlca.201200610)
B. M. Šmit, R. Z. Pavlović, D. A. Milenković, Z. S. Marković, Beilstein J. Org. Chem. 11 (2015) 1865 (https://dx.doi.org/10.3762/bjoc.11.200).