An inverse gas chromatography study of the adsorption of organics on zeolite and zeolite/iron oxyhydroxide composite at the infinite and finite surface coverage Scientific paper
Main Article Content
Abstract
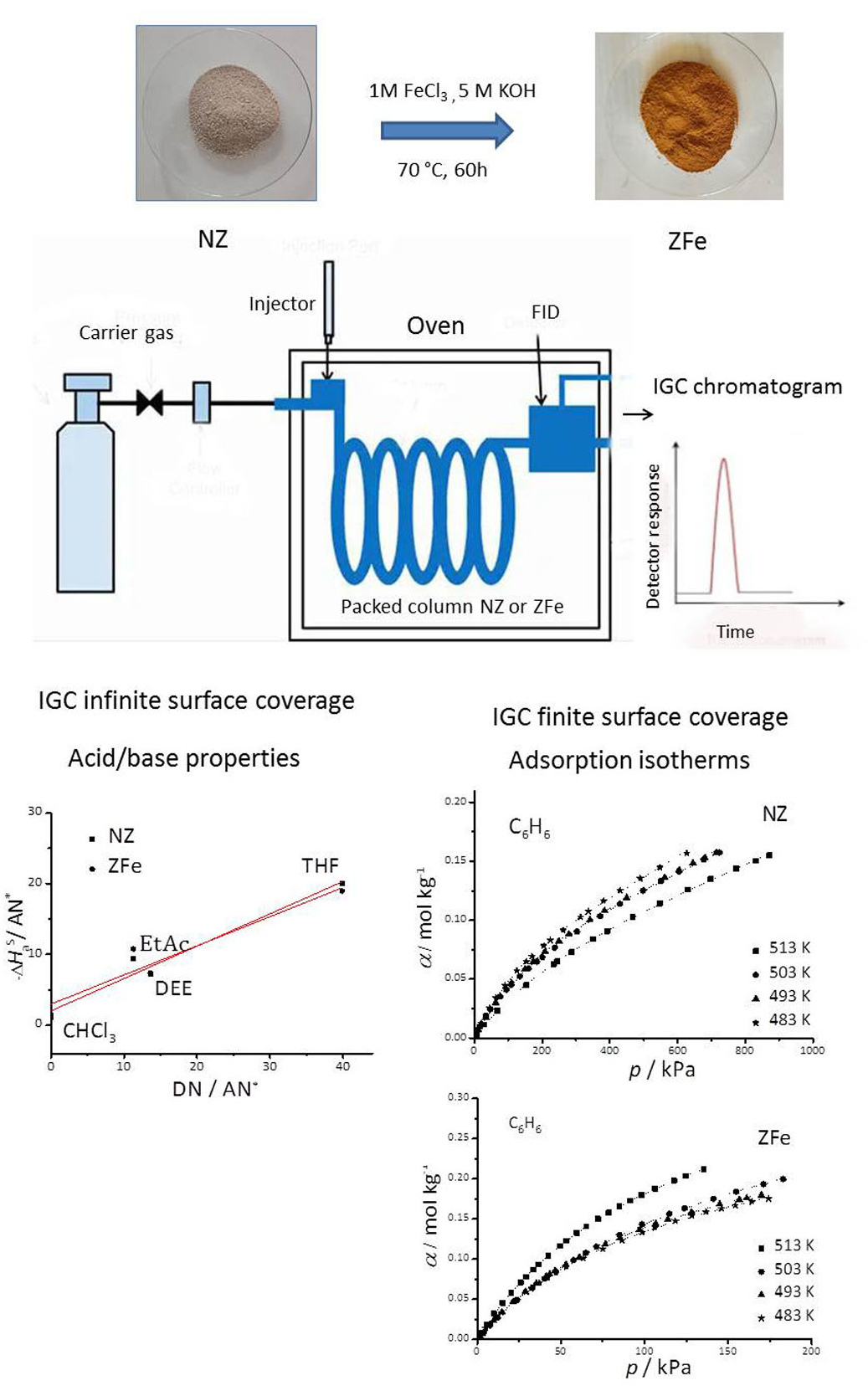
The surfaces of natural (NZ) and zeolite/iron oxyhydroxide composite (ZFe) samples were analysed by means of inverse gas chromatography (IGC) using the adsorption data of organic non-polar and polar probes, in the infinite and finite-dilution regimes, in the temperature range 483–513 K. The dispersive components of the free energy of adsorption, γS, determined by the Gray method, decreased with increasing temperature for both zeolites. The specific interactions were characterised by the specific free adsorption energy change, ΔGaS, the specific enthalpy change of adsorption, ΔHaS, as well as the donor and acceptor interaction parameters (KA, KD) and the basic character of the NZ and ZFe was evidenced. The adsorption isotherms of n-hexane, benzene, chloroform and tetrahydrofuran (THF) were determined under finite surface coverage and used to estimate the specific surface area and the adsorption energy distribution. The adsorption capacity of the ZFe was higher than for NZ for all the investigated adsorbates. The specific surface areas and pore size distributions were also determined using nitrogen adsorption–desorption isotherms, i.e., the BET method. It was observed that the nature of the adsorbate and the properties of the solid surface of the initial and modified samples governed the uptake of adsorbates.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200135
References
M.O. Daramola, E.F. Aransiola, T.V. Ojumu, Materials 5 (2012) 2101 (https://doi.org/10.3390/ma5112101)
N. Finish, P. Ramos, E. J.C. Borojovich, O. Zeiri, Y. Amar, M. Gottlieb, J. Hazard. Mater. 457 (2023) 131784 (https://doi.org/10.1016/j.jhazmat.2023.131784
D. Nibou, H. Mekatel, S. Amokrane, M. Barkat, M. Trari, J. Hazard. Mater. 173 (2010) 637 (https://doi.org/10.1016/j.jhazmat.2009.08.132)
V. Yadav, L. Kumar, N. Saini, M. Yadav, N. Singh, V. Murugasen, E. Varathan, Water Air Soil Poll. 234 (2023) 435 (https://doi.org/10.1007/s11270-023-06469-4)
S. Wang, Y. Peng, Chem. Eng. J. 156 (2010) (https://doi.org/10.1016/j.cej.2009.10.029)
K. Shikhaliyev, T. Onsree, A. H. Jaeschke, S. M. Ghoreishian, K. Shariati, A. Martinez, A. Katz, S. Hwang, A. Gaffney, J. M. Urban-Klaehn, J. Lauterbach, Appl. Catal., B 337 (2023) 122991 (https://doi.org/10.1016/j.apcatb.2023.122991)
M.A. Sadenova, S.A. Abdulina, S. A. Tungatarova, Clean Technol. Environ. 18 (2016) 449 (https://doi.org/10.1007/s10098-015-1018-6)
M. Kuronen, M. Weller, R. Townsend, R. Harjula, React. Funct. Polym. 66 (2006) 1350 (https://doi.org/10.1016/j.reactfunctpolym.2006.03.019)
I. Rodriguez-Iznaga, V. Petranovskii, G.Rodriguez-Fuentes, J. Environ. Chem. Eng. 2 (2014) 1221 (https://doi.org/10.1016/j.jece.2014.05.012)
M. Doula, Water Res. 40 (2006) 3167 (https://doi.org/10.1016/j.watres.2009.05.037)
M. Doula, A. Dimirkou, J. Hazard. Mater. 151 (2008) 738 (https://doi.org/10.1016/j.jhazmat.2007.06.047)
A. Dimirkou, Water Res. 41 (2007) 2763 (https://doi.org/10.1016/j.watres.2007.02.045)
M. Kragović, A. Daković, Ž. Sekulić, M. Trgo, M. Ugrina, J. Perić, G. D. Gatta, Appl. Surf. Sci. 258 (2012) 3667 (https://doi.org/10.1016/j.apsusc.2011.12.002)
P. Praipipat, S. Jangkorn, P. Ngamsurach, Environ. Nanotechnol. Monit. 20 (2023) 100812 (https://doi.org/10.1016/j.enmm.2023.100812)
M. T. Mihajlović, S. S. Lazarević, I. M. Janković-Častvan, B. M. Jokić, Đ. T. Janaćković, R. D. Petrović, Chem. Ind. Chem. Eng. Q. 20 (2014) 283 (https://doi.org/10.2298/CICEQ121017010M)
M. T. Mihajlović, S. S. Lazarević, I. M. Janković-Častvan, J. Kovač, B. M. Jokić, Đ. T. Janaćković, R. D. Petrović, Clean Technol. Environ. Policy 17 (2015) 407 (https://doi.org/10.1007/s10098-014-0794-8)
P. M. Nekhunguni, N. T. Tavengwa, H. Tutu, J. Environ. Manage. 197 (2017) 550 (https://doi.org/10.1016/j.jenvman.2017.04.038)
A. Badeenezhad, A. Azhdarpoor, S. Bahrami, S. Yousefinejad, Mol. Simulat. 45 (2019) 564 https://doi.org/10.1080/08927022.2018.1564077
N. J. Singh, B. Wareppam, A. Kumar, K. P. Singh, V. K. Garg, A. C. Oliveira, L. H. Singh, J. Mater. Res. 38 (2023) 1149 (https://doi.org/10.1557/s43578-022-00859-w)
S. Mohammadi-Jam, K.E. Waters, Adv. Colloid Interf. Sci. 212 (2014) 21 (https://doi.org/10.1016/j.cis.2014.07.002)
J. P. Jolivet, E. Tronc, Corinne Chaneac, C. R. Geoscience 338 (2006) 488 (https://doi.org/10.1016/j.crte.2006.04.014)
F. Rouquerol, J. Rouquerol, K. Sing, Adsorption by Powders and Porous Solids, Academic Press, London, 1999 (https://doi.org/10.1016/B978-0-12-598920-6.X5000-3)
E. P. Barrett, L. G. Joyner, P. P. Halenda, J. Am. Chem. Soc. 73 (1951) 373 (http://dx.doi.org/10.1021/ja01145a126)
B. C. Lippens, J. H. de Boer, J. Catal. 4 (1965) 319 (https://doi.org/10.1016/0021-9517(65)90307-6)
U. Kuila, M. Prasad, Geophys. Prospec. 61 (2013) 341 (https://doi.org/10.1111/1365-2478.12028)
E. Diaz, S. Ordonez, A. Vega, J. Coca, J. Chromatogr., A 1049 (2004) 139 (https://doi.org/10.1016/j.chroma.2004.07.061)
C. Bilgic, F.Tumsek, J. Chromatogr., A 1162 (2007) 83 (https://doi.org/10.1016/j.chroma.2007.04.003)
E. Diez, J.M Gomez, A. Rodriguez, A. Martinez, P. Saez, Environ. Prog. Sustain. 39 (2020) e13412 (https://doi.org/10.1002/ep.13412)
Y. J. Huang, Y. Jiang, V. R. R. Marthala, B. Thomas, E. Romanova, M. Hunger, Phys. Chem., C 112 (2008) 381 (https://doi.org/10.1021/jp7103616).