Hydroxyapatite/nifuroxazide conjugate: Characterization, drug release and antimicrobial activity Scientific paper
Main Article Content
Abstract
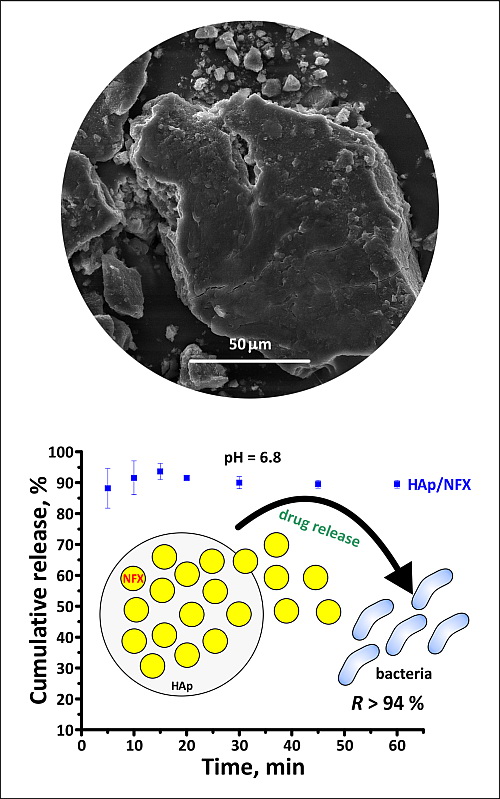
Synthetic hydroxyapatite (Ca10(PO4)6(OH)2, HAp) is very similar to the inorganic part of the bones and teeth of mammals. It is a well-known biomaterial with good biocompatibility, osteoconductivity and bioactivity. Nifuroxazide (C12H9N3O5, NFX) is a broad-spectrum antibacterial drug and poorly soluble in water. In order to increase the solubility of NFX, nano-sized HAp powder and raw NFX drug were mixed giving, as a result, a HAp/NFX conjugate. Characterization of the raw materials and the obtained conjugate confirmed the integration of NFX on the HAp surface. An in vitro study of drug release in simulated stomach acid and intestinal fluid showed a much faster release of NFX from HAp surface than those of the raw drug. The HAp/NFX conjugate showed excellent inhibitory effects against Gram-positive bacterium Staphylococcus aureus, Gram-negative bacterium Escherichia coli and yeast Candida albicans, proving the nano-sized HAp powder as a promising drug carrier.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. V. Dorozhkin, Hydroxyapatite and Other Calcium Orthophosphates, Nova Publisher, New York, 2017 (ISBN: 978-1-53611-897-1)
J. Klesing, S. Chernousova, M. Epple, J. Mater. Chem. 22 (2012) 199 (https://dx.doi.org/10.1039/C1JM13502C)
T. Matsumoto, M. Okazaki, M. Inoue, S. Yamaguchi, T. Kusunose, T. Toyonaga, Y. Hamada, J. Takahashi, Biomaterials 25 (2004) 3807 (https://dx.doi.org/10.1016/j.biomaterials.2003.10.081)
T. S. P. Cellet, G. M. Pereira, E. C. Muniz, R. Silva, A. F. Rubira, J. Mater. Chem., B 3 (2015) 6837 (https://dx.doi.org/10.1039/c5tb00856e)
Q. Zhao, T. Wang, J. Wang, L. Zheng, T. Jiang, G. Cheng, S. Wang, Appl. Surf. Sci. 257 (2011) 10126 (https://dx.doi.org/10.1016/j.apsusc.2011.06.161)
Y. Ryabenkova, N. Jadav, M. Conte, M. F. A. Hippler, N. Reeves-McLaren, P. D. Coates, P. Twigg, A. Paradkar, Langmuir 33 (2017) 2965 (https://dx.doi.org/10.1021/acs.langmuir.6b04510)
A. L. C. Maia, C. de Aguiar Ferreira, A. L. B. deBarros, A. T. M. e Silva, G. A. Ramal-des, A. da Silva Cunha Júnior, D. C. de Pádua Oliveira, C. Fernandes, D. C. F. Soares, J. Drug Target. 26 (2018) 592 (https://dx.doi.org/10.1080/1061186X.2017.1401078)
D. Loca, J. Locs, A. Dubnika, V. Zalite, L. Berzina-Cimdina, in Hydroxyapatite (HAp) for Biomedical Applications, М. Mucalo, Еd., 1st ed., Woodhead Publishing, Cambridge, 2015, pp.189–209 (Hardcover ISBN: 9781782420330)
Z. Yang, W. Xu, M. Ji, A. Xie, Y. Shen, M. Zhu, Eur. J. Inorg. Chem. (2017) 5621 (https://dx.doi.org/10.1002/ejic.201701081)
C. Bailly, Drug Discov. Today 24 (2019) 1930 (https://dx.doi.org/10.1016/j.drudis.2019.06.017)
L. Luo, F. Xu, H. Peng, Y. Luo, X. Tian, G. Battaglia, H. Zhang, Q. Gong, Z. Gu, K. Luo, J. Control. Release 318 (2020) 124 (https://dx.doi.org/10.1016/j.jconrel.2019.12.017)
R. T. Peterson, Cell Chem. Biol. 25 (2018) 1439 (https://dx.doi.org/10.1016/j.chembiol.2018.12.005)
N. H. Zuma, J. Aucamp, D. D. N'Da, Eur. J. Pharm. Sci. 140 (2019) 105092 (https://dx.doi.org/10.1016/j.ejps.2019.105092)
L. Chen, H. Zhu, S. Yang, B. Zhou, F. You, X. Yan, Mater. Lett. 143 (2015) 252 (https://dx.doi.org/10.1016/j.matlet.2014.12.118)
Ž. Radovanović, A. M. Kazuz, P. Vulić, L. Radovanović, Đ. Veljović, R. Petrović, Đ. Janaćković; in Proceedings of 6th International Conference on Electrical, Electronic and Computing Engineering (Ic)ETRAN (2019), Silver Lake, Serbia, Proceedings_IcETRAN_ETRAN_2019, ETRAN Society, Belgrade, Academic Mind, Belgrade, 2019, p. 676 (https://etran.rs/2019/Proceedings_IcETRAN_ETRAN_2019.pdf)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystаllogr., B 72 (2016) 171 (https://dx.doi.org/10.1107/S2052520616003954)
M. I. Toral, M. Paine, P. Leyton, P. Richter, J. AOAC Int. 87 (2004) 1323 (https://dx.doi.org/10.1093/jaoac/87.6.1323)
N. S. Sambudi, S. Cho, K. Cho, RSC Adv. 6 (2016) 43041 (https://dx.doi.org/10.1039/C6RA03147A)
T. Bera, A. N. Vivek, S. K. Saraf, P. Ramachandrarao, Biomed. Mat. 3 (2008) 025001 (https://dx.doi.org/10.1088/1748-6041/3/2/025001)
M. Ponjavić, M. S. Nikolić, J. Nikodinović-Runić, T. Ilić-Tomić, J. Djonlagić, Int. J. Polym. Mater. 68 (2019) 308 (https://dx.doi.org/10.1080/00914037.2018.1445631).