Cobalt catalyzed defunctionalization reactions Review article
Main Article Content
Abstract
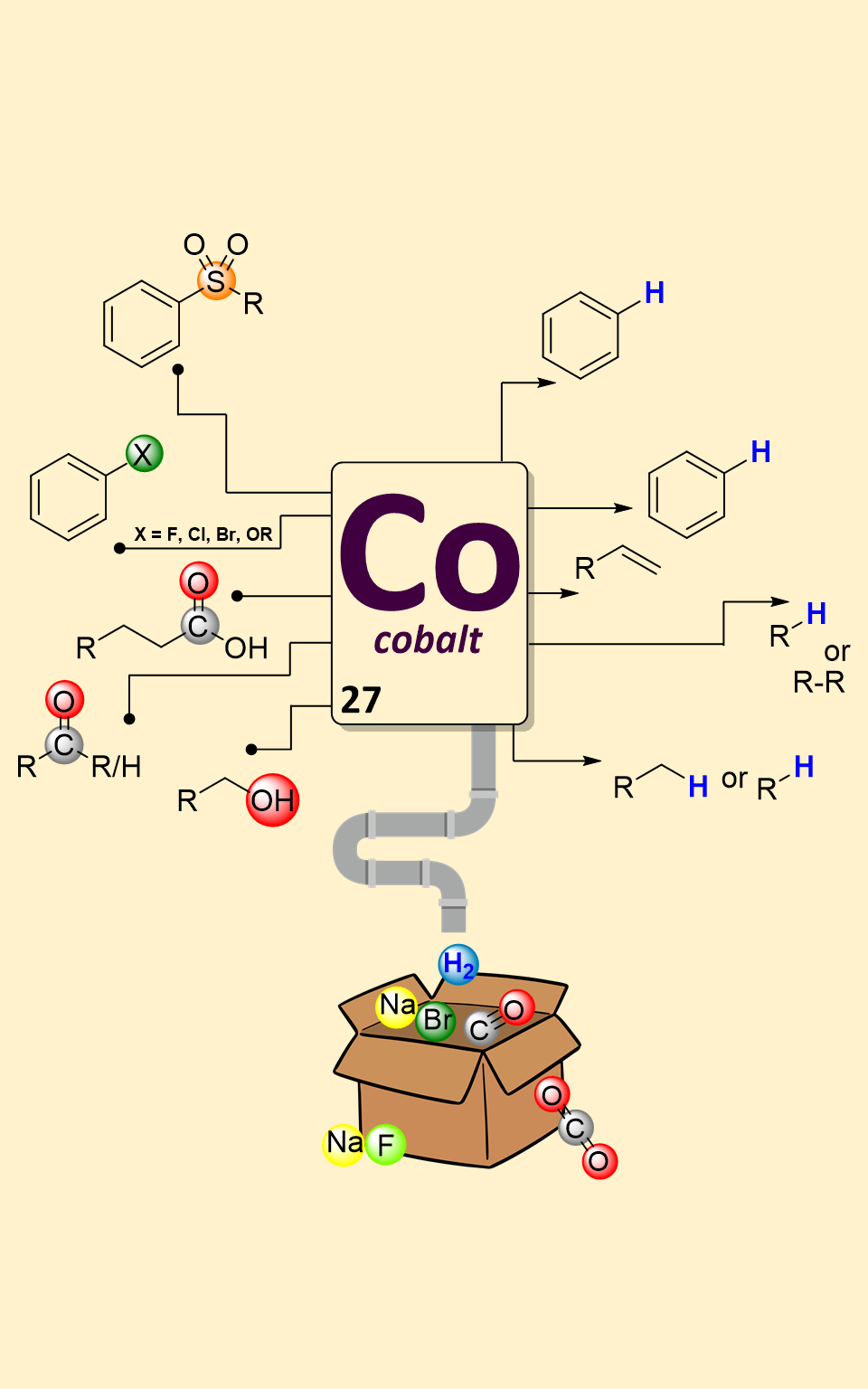
Catalytic defunctionalization of complex molecules has attracted significant attention in organic synthesis. This reaction enables common functional groups to serve as “traceless handles” for the new bond construction. In this mini-review, we have summarized the latest advances, methodologies and mechanistic insights into the selective cleavage of C–C and C–X bonds catalysed by cobalt complexes, shedding light on their increasing importance in modern chemical synthesis. The content of this review is categorized according to the type of functional group being removed from molecules.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200288
References
A. Modak, D. Maiti, Org. Biomol. Chem. 14 (2016) 21 (https://doi.org/10.1039/C5OB01949D)
Ž. Selaković, A. M. Nikolić, V. Ajdačić, I. M. Opsenica, Eur. J. Org. Chem. (2022) e202101265 (https://doi.org/10.1002/ejoc.202101265)
J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius, B. M. Weckhuysen, Chem. Rev. 110 (2010) 3552 (https://doi.org/10.1021/cr900354u)
J. S. Dickstein, J. M. Curto, O. Gutierrez, C. A. Mulrooney, M. C. Kozlowski, J. Org. Chem. 78 (2013) 4744 (https://doi.org/10.1021/jo400222c)
O. Navarro, H. Kaur, P. Mahjoor, S. P. Nolan, J. Org. Chem. 69 (2004) 3173 (https://doi.org/10.1021/jo035834p)
J. F. Hooper, R. D. Young, A. S. Weller, M. C. Willis, Chem. Eur. J. 19 (2013) 3125 (https://doi.org/10.1002/chem.201204056)
M. Tobisu, R. Nakamura, Y. Kita, N. Chatani, J. Am. Chem. Soc. 131 (2009) 3174 (https://doi.org/10.1021/ja810142v)
N. Chatani, H. Tatamidani, Y. Ie, F. Kakiuchi, S. Murai, J. Am. Chem. Soc. 123 (2001) 4849 (https://doi.org/10.1021/ja0103501)
G. Domazetis, B. Tarpey, D. Dolphin, B. R. James, J. Chem. Soc., Chem. Commun. (1980) 939 (https://doi.org/10.1039/C39800000939)
B. Kokić, B. Vulović, M. Jović, A. Andrijević, V. Ajdačić, I. M. Opsenica, Eur. J. Org. Chem. 26 (2023) e202300997 (https://doi.org/10.1002/ejoc.202300997)
B. Kokić, Ž. Selaković, A. M. Nikolić, A. Andrijević, B. Anđelković, V. Ajdačić, I. M. Opsenica, Eur.J. Org.Chem. (2022) e202201112 (https://doi.org/10.1002/ejoc.202201112)
G. R. Lappin, J. D. Sauer, Alpha Olefns Applications Handbook, Marcel Dekker, New York, 1989 (ISBN:9780824778958)
I. T. Horváth, Chem. Rev. 118 (2018) 369 (https://doi.org/10.1021/acs.chemrev.7b00721)
R. A. Sheldon, J. K. Kochi, Org. React. 19 (2011) 279 (https://doi.org/10.1002/0471264180.or019.04)
T.Takeda, Modern Carbonyl Olefination: Methods and Applications; John Wiley & Sons, Hoboken, NJ, 2006 (ISBN:978-3-527-60538-5)
L. J. Gooßen, N. A. Rodríguez, Chem. Commun. (2004) 724 (https://doi.org/10.1039/B316613A)
A. John, M. O. Miranda, K. Ding, B. Dereli, M. A. Ortuño, A. M. LaPointe, G. W. Coates, C. J. Cramer, W. B. Tolman, Organometallics 35 (2016) 2391 (https://doi.org/10.1021/acs.organomet.6b00415)
A. John, M. A. Hillmyer, W. B. Tolman, Organometallics 36 (2017) 506 (https://doi.org/10.1021/acs.organomet.6b00940)
S. Maetani, T. Fukuyama, N. Suzuki, D. Ishihara, I. Ryu, Organometallics 30 (2011) 1389 (https://doi.org/10.1021/om1009268)
K. C. Cartwright, J. A. Tunge, ACS Catal. 8 (2018) 11801 (https://doi.org/10.1021/acscatal.8b03282)
K. C. Cartwright, E. Joseph, C. G. Comadoll, J. A. Tunge, Chem. Eur. J. 26 (2020) 12454 (https://doi.org/10.1002/chem.202001952)
X. Sun , J. Chen , T. Ritter , Nature Chem. 10 (2018) 1229 (https://doi.org/10.1038/s41557-018-0142-4)
V. T. Nguyen, V. D. Nguyen, G. C. Haug, H. T. Dang, S. Jin, Z. Li, C. Flores-Hansen, B. S. Benavides, H. D. Arman, O. V. Larionov, ACS Catal. 9 (2019) 9485 (https://doi.org/10.1021/acscatal.9b02951)
E. N. G. Marsh, M. W. Waugh, ACS Catal. 3 (2013) 2515 (https://doi.org/10.1021/cs400637t)
N. Terzić Jovanović, V. Ajdačić, J. Serb. Chem. Soc. 87 (2022) 669 (https://doi.org/10.2298/JSC220128024T)
H. Lu, T. Y. Yu, P. F. Xu, H. Wei, Chem. Rev. 121 (2021) 365 (https://doi.org/10.1021/acs.chemrev.0c00153)
S. Yuan, H. Sun, S. Zhang, X. Li, Inorg. Chim. Acta 439 (2016) 100 (https://doi.org/10.1016/j.ica.2015.10.006)
D. J. Abrams, J. G. West, E. J. Sorensen, Chem. Sci. 8 (2017) 1954 (https://doi.org/10.1039/C6SC04607J)
H. Alawisi, K. F. Al-Afyouni, H. D. Arman, Z. J. Tonzetich, Organometallics 37 (2018) 4128 (https://doi.org/10.1021/acs.organomet.8b00668)
30. D. Kolb, M. Morgenstern, B. König, Chem. Commun. 59 (2023) 8592 (https://doi.org/10.1039/D3CC02170J)
D. Kolb, A. A. Almasalma, M. Morgenstern, L. Ganser, I. Weidacher, B. König, ChemPhotoChem 7 (2023) e202300167 (https://doi.org/10.1002/cptc.202300167)
A. Dobbs, J. Org. Chem. 66 (2001) 638 (https://doi.org/10.1021/jo0057396)
A. Ramanathan, L. S. Jimenez, Synthesis (2010) 217 (https://doi.org/10.1055/s-0029-1217112)
S. Agarwal, S. R. Al-Abed, D. D. Dionysiou, Environ. Sci. Technol. 41 (2007) 3722 (https://doi.org/10.1021/es062886y)
A. Kokanović, V. Ajdačić, N. Terzić Jovanović, S. Stankic, I. M. Opsenica, ACS Appl. Nano Mater. 6 (2023) 15820 (https://doi.org/10.1021/acsanm.3c02743)
J. Li, T. Zheng, H. Suna, X. Li, Dalton Trans. 42 (2013) 13048 (https://doi.org/10.1039/C3DT50409C)
K. S. Chan, C. R. Liu, K. L. Wong, Tetrahedron Lett. 56 (2015) 2728 (https://doi.org/10.1016/j.tetlet.2015.04.014)
C. Chen, H. Zuo, K. S. Chan, Tetrahedron 75 (2019) 510 (https://doi.org/10.1016/j.tet.2018.12.010)
R. Z. Liao, S. L. Chen, P. E. M. Siegbahn, ACS Catal. 5 (2015) 7350 (https://doi.org/10.1021/acscatal.5b01502)
B. Sahoo, A. E. Surkus, M. M. Pohl, J. Radnik, M. Schneider, S. Bachmann, M. Scalone, K. Junge, M. Beller, Angew. Chem. Int. Ed. 56 (2017) 11242 (https://doi.org/10.1002/anie.201702478)
B. R. P. Reddy, A. Auffrant, C. Gosmini, Asian J. Org. Chem. 10 (2021) 3275 (https://doi.org/10.1002/ajoc.202100616)
K. M. McCauley, D. A. Pratt, S. R. Wilson, J. Shey, T. J. Burkey, W. A. van der Donk, J. Am. Chem. Soc. 127 (2005) 1126 (https://doi.org/10.1021/ja048573p)
43. H. Shimakoshi, M. Tokunaga, T. Babaa, Y. Hisaeda, Chem. Commun. (2004) 1806 (https://doi.org/10.1039/B406400C)
H. Shimakoshi, Y. Hisaeda, Chem. Rec. 21 (2021) 2080 (https://doi.org/10.1002/tcr.202100077)
H. Shimakoshi, Y. Hisaeda, ChemPlusChem 82 (2017) 18 (https://doi.org/10.1002/cplu.201600303)
J. M. Herrmann, B. König, Eur. J. Org. Chem. (2013) 7017 (https://doi.org/10.1002/ejoc.201300657)
M. Tobisu, K. Yamakawa, T. Shimasakia, N. Chatani, Chem. Commun. 47 (2011) 2946 (https://doi.org/10.1039/C0CC05169A)
T. Funabiki, Y. Yamazaki, K. Tarama, J. Chem. Soc., Chem. Commun. (1978) 63 (https://doi.org/10.1039/C39780000063)
J. T. Lee, H. Alper, Tetrahedron Lett. 31 (1990) 4101 (https://doi.org/10.1016/S0040-4039(00)97553-1)
B. H. Gross, R. C. Mebane, D. L. Armstrong, Appl. Catal., A 219 (2001) 281 (https://doi.org/10.1016/S0926-860X(01)00700-1)
Y. L. Ren, M. Tian, X. Z. Tian, Q. Wang, H. Shang, J. Wang, Z. C. Zhang, Catal. Commun. 52 (2014) 36 (https://doi.org/10.1016/j.catcom.2014.03.036)
J. Fukuda, K. Nogi, H. Yorimitsu, Asian J. Org. Chem. 7 (2018) 2049 (https://doi.org/10.1002/ajoc.201800473).