Introductory concept for teaching chirality – Symmetry of the asymmetric Scientific paper
Main Article Content
Abstract
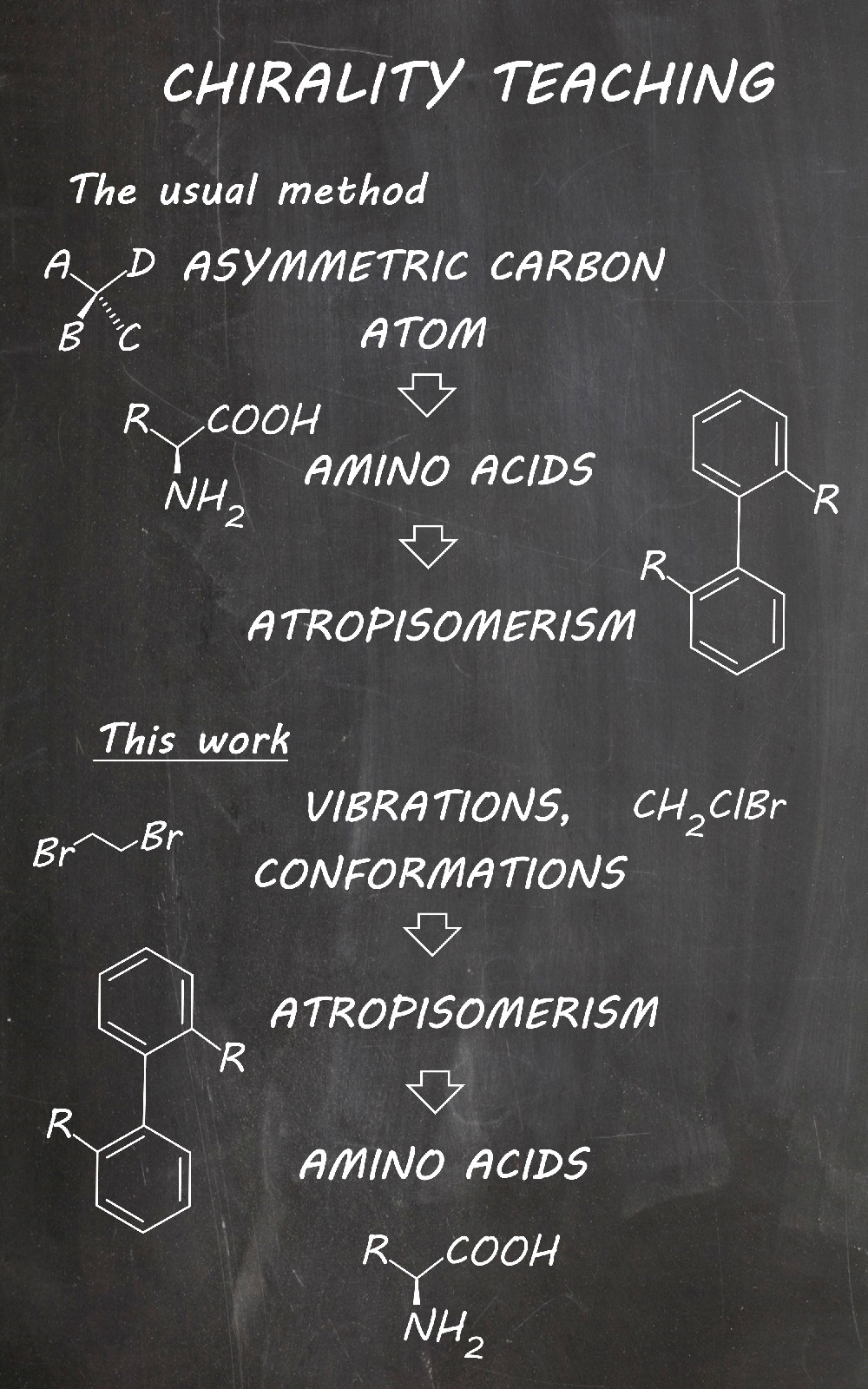
Chirality is traditionally a problematic subject for undergraduate students at the beginning of learning organic chemistry, yet it is of great importance in life sciences. If the initial introduction of chirality is conducted carelessly, students will face ambiguity through the rest of the course and education every time they come across chirality-related subjects. Although there are numerous methods for overcoming the problems of visualization of chiral molecules in 3D space, the connection of chirality with molecular changes like vibrations and conformations is usually not explained thoroughly. In this work, chirality is introduced on dynamic (real) systems, because students from the start should perceive molecules in their natural state of constant motion and change. Apart from the proposition of the lecture concept, exercises are also included, that employ free and readily available software.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200168;451-03-47/2023-01/200288
References
L. J. Juszczak, J. Chem. Educ. 98 (2021) 3384 (https://doi.org/10.1021/acs.jchemed.1c00633)
a) A. Alsfouk, J. Chem. Educ. 99 (2022) 1900 (https://doi.org/10.1021/acs.jchemed.1c01267); b) C. E. Dickenson, R. A. R. Blackburn, R. G. Britton, J. Chem. Educ. 97 (2020) 3714 (https://doi.org/10.1021/acs.jchemed.0c00457); c) M. W. Pelter, M. T. Howell, C. Anderson, A. Sayeed, J. Chem. Educ. 97 (2020) 754 (https://doi.org/10.1021/acs.jchemed.9b00934); d) D. M. Silva, C. M. R. Ribeiro, J. Chem. Educ. 94 (2017) 1272 (https://doi.org/10.1021/acs.jchemed.7b00219);
e) M. Abraham, V. Varghese, H. Tang, J. Chem. Educ. 87 (2010) 1425 (https://doi.org/10.1021/ed100497f); f) P. Lloyd-Williams, E. Giralt, J. Chem. Educ. 82 (2005), 1031 (https://doi.org/10.1021/ed082p1031)
G. P. Moss, Pure Appl. Chem. 68 (1996) 2193 (https://doi.org/10.1351/pac199668122193)
a) A. Guijarro, M. Yus, The Origin of Chirality in the Molecules of Life: A Revision from Awareness to the Current Theories and Perspectives of this Unsolved Problem, The Royal Society of Chemistry, Cambridge, 2009, pp. 31–48 (ISBN: 9780854041565; b) M. Quack, G. Seyfang, G. Wichmann, Chem. Sci. 13 (2022) 10598 (https://doi.org/10.1039/D2SC01323A) and references therein
C. J. M. Stirling, J. Chem. Educ. 51 (1974) 50 (https://doi.org/10.1021/ed051p50)
P. Lloyd-Williams, E. Giralt, J. Chem. Educ. 80 (2003) 1178 (https://doi.org/10.1021/ed080p1178)
D. A. Dougherty, E. V. Anslyn, Modern Physical Organic Chemistry, University Science Books, Herndon, VA, 2006 (ISBN: 9781891389313)
Y. Zhang, M. Sayama, M. Luo, Y. Lu, D. J. Tantillo, J. Chem. Educ. 99 (2022) 2721 (https://doi.org/10.1021/acs.jchemed.2c00443)
a) B. M. Wong, M. M. Fadri, S. Raman, J. Comput. Chem. 29 (2007) 481 (https://doi.org/10.1002/jcc.20807); b) D. Nori-Shargh, J. E. Boggs, Struct. Chem. 22 (2010) 253 (https://doi.org/10.1007/s11224-010-9675-x)
a) D. J. Brand, J. Fisher, J. Chem. Educ. 64 (1987) 1035 (https://doi.org/10.1021/ed064p1035); b) M. Čepič, Mol. Cryst. Liq. Cryst. 475 (2007) 151 (https://doi.org/10.1080/15421400701681141)
V. Schurig, S. Reich, Chirality 10 (1998) 316 (https://doi.org/10.1002/(SICI)1520-636X(1998)10:4<316::AID-CHIR5>3.0.CO;2-5)
A. Mazzanti, L. Lunazzi, M. Minzoni, J. E. Anderson, J. Org. Chem. 71 (2006) 5474 (https://doi.org/10.1021/jo060205b)
L. Schaefer, C. Van Alsenoy, L. Van Den Enden, J. Chem. Educ. 61 (1984) 945 (https://doi.org/10.1021/ed061p945) and references therein
J. M. Lehn, in Dynamic Stereochemistry, J. E. Baldwin, R. H. Fleming, J. M. Lehn, W. Tochtermann, Eds., Springer, Berlin, 1970, p. 311 (https://doi.org/10.1007/BFb0050820)
I. Fernández, N. Khiar, Chem. Rev. 103 (2003) 3651 (https://doi.org/10.1021/cr990372u)
a) A. Messara, N. Vanthuyne, P. Diter, M. Elhabiri, A. Panossian, G. Hanquet, E. Magnier, F. R. Leroux, Eur. J. Org. Chem. 2021 (2021) 5019 (https://doi.org/10.1002/ejoc.202100816) b) H. Marom, P. U. Biedermann, I. Agranat, Chirality 19 (2007) 559 (https://doi.org/10.1002/chir.20417)
J. L. Bada, Interdiscip. Sci. Rev. 7 (1982) 30 (https://doi.org/10.1179/030801882789801304)
E. E. Eliel, S. H. Wilen, L. N. Mander, Stereochemistry of Organic Compounds, Wiley-Interscience, Hoboken, NJ, 1994, pp. 1119–1190 (ISBN: 9780471016700)
a) K. Mislow, P. Bickart, Isr. J. Chem. 15 (1976) 1 (https://doi.org/10.1002/ijch.197600002); b) D. F. Mowery, Jr. J. Chem. Educ. 46 (1969) 269 (https://doi.org/10.1021/ed046p269).